Immune stimulation may treat depression via cytokines that boost neurotrophic factors | Charles Raison
Get the full length version of this episode as a podcast.
This episode will make a great companion for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
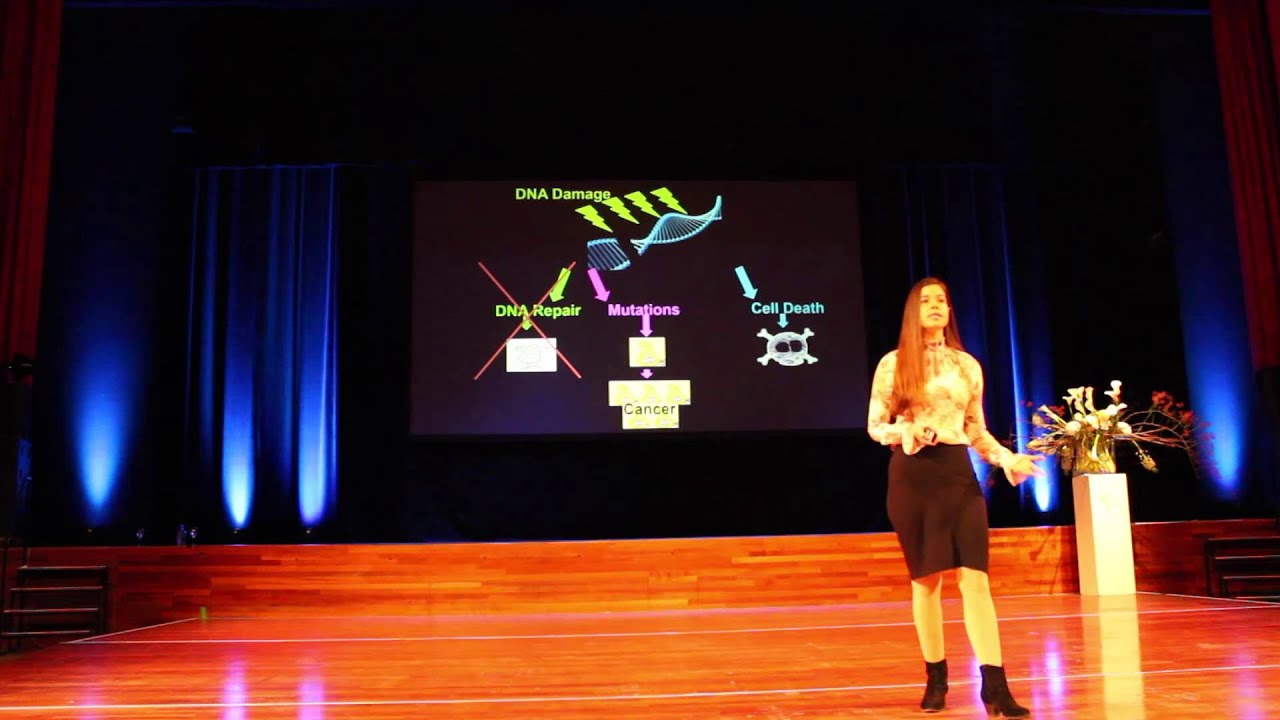
A key player in the pathophysiology of depression is inflammation, a critical element of the body's immune system. Inflammation is a conserved biological response that developed during humans' ancient past, when regular exposure to pathogens dictated highly coordinated behavioral and immune responses to ensure survival. The fallout of these responses is an "inflammatory bias"– a propensity for the body to launch an indiscriminate response to a stressor, regardless of its source. Elevated biomarkers of inflammation, which are commonly observed in people who have depression, chronically activate the body's inflammatory response system, promoting the development of depressive symptoms and inducing changes in brain and neuroendocrine function. Interestingly, acute activation of the immune system can temporarily reduce inflammation and its accompanying depressive symptoms. In this clip, Dr. Charles Raison describes the complex relationship between inflammation and depression.
- Charles: But what’s interesting is there’s a counter, there is a little bit of counter data. This was done years ago in Germany where they actually took people that were catastrophic, it’s a small study, but they took people that had been inpatient, catastrophically depressed, and they shot them up with endotoxin and it produced a powerful antidepressant response. Now, what’s interesting about that is that there’s a relevant animal study from Raz Yirmiya in Israel, where they took mice, and I’m pretty sure it’s mice, not rats, and subjects them with this 20-day horrible stressor. They showed that the stressor crazy activates inflammation, leads to apoptosis, death of microglial cells in the brain and, you know, huge anxious depressive behavior afterwards, right? So what’s interesting was they showed that if you block the inflammation right before the start of the stressor, sort of it starts, you block it, you can prevent the apoptosis, you can prevent the downstream behavioral effects, it’s protective. If you do nothing here and you let the little rodents go through the horrible stressor and you block the inflammation afterwards, they do worse. If you stimulate inflammation, they get antidepressant response. So there’s a little bit of a background, that I am just one of the few people, but there are some of us that are interested in this idea that inflammation is a funny thing, right? So these cytokines, these classic inflammatory molecules like TNF, Tumor Necrosis Factor Alpha, IL1 Beta, IL6, at lower levels in the brain, they actually have neurotrophic effects.
- Rhonda: Kinda like a hormetic stressor, where they’re active?
- Charles: We don’t know, is it stressor, or is it just that they evolved? Nature is so cheap, you know, it always wants to reuse things. That’s what makes things, evolutionary processes do this constantly, and that’s why things are, one of the reasons why biological systems are hard to understand. You know, if they’ve generated TNF knock-out mice they, can’t find their way out of a bag, they are as dumb as dirt. There’s something about lower levels of these mediators that may actually be beneficial in the CNS, at least. And then there’s sort of a U, and then all of a sudden man, very, very rapidly, they become what we think of us as counterproductive. You know, they become depressive inducing, they cause tissue damage. Now, they evolve for a purpose. I mean, if that was just a negative thing, that would not happen. I mean, life is such a rough competitive game. It may be the case that the reason that you get the sort of CNS inflammation from, either from peripheral cells coming into the CNS, or from these resident macrophage-type CNS cells being activated. It’s probably a way of reducing the risk of pathogen manipulation, where you basically, because it’s interesting, if you look at what...when inflammation gets activated in the CNS, it has a trophic effect. It doesn’t just go everywhere. It tends to go to an area called the Cingulate Cortex, and the dopamine area is down in the Ventral striatum. We and others have suggested that it may be a way trying to take these areas offline so that they’re not able to be manipulated by pathogens. You know, you don’t necessarily want bugs driving your system. We now know that many of these sort of CNS organisms, like [inaudible 00:24:33], you know, the thing that, no, it will come to me, that drives crazy dopamine behavior, that a lot of times, these microorganisms will actually change behavior in ways that benefit their survival and reproduction.
- Rhonda: Toxoplasmosis.
- Charles: Yeah, toxo. Right. There’s probably an evolutionary reason for why you see the U shape curve, but that may also explain why, you know, we’ve been working on the idea that people have been really, really chronically depressed. So if you look at people that are chronically depressed, and we talked about the fact that, you know, inflammation is elevated in depression, it’s true, but it’s only true in a certain way. So what you really see is, you know, for any inflammatory biomarker, here’s where it’s at if you’re healthy, here’s where it’s at if you have the flu, or you got rheumatoid arthritis, right? If you’re healthy, here’s where it’s at, and if you’re healthy and depressed, it’s here, right? Now, day in, day out, day in, day out, that’s enough to set you up for every evil thing: heart attack, strokes, dementia, I mean, because it’s a gradual wear and tear. But then if you look more closely, what you really see is this, so that there’s a huge overlap between depressed people and not depressed people. So there’s lots of depressed people that are desperately depressed that have low levels of inflammation, and it’s only some that are elevated. Now I thought, for many years, because I’m kind of a lumper, not a splitter, that may be what you were looking at here was that depressed people, that they’re all in inflammatory simple thing, that some depressed people just have higher inflammation, and that’s what’s doing it. And other people may be depressed because they’re more sensitive to inflammation, but that it’s all too much inflammation in one way or other, you know. We now are pretty sure that that’s not true, that in fact the reason that depression is associated with increased inflammation is because there’s a subgroup of depressed people that have elevated inflammation, and they’re different than depressed people that don’t. This is the work of my mentor, Andy Miller, in the last five or seven years. They’ve just been world leader showing that if you take regular old, depressed people, he got like 250 of them and did this amazing series of studies. People that have, there’s not a cut off, but the people that have higher levels of inflammation and depressed, have different functional connectivity in their brains than people that have lower levels. We showed, Andy and I showed years earlier that they also have very different responses to immune agents than people that have lower levels of inflammation. So I think in fact, that there’s a subgroup of very depressed people that might benefit from a kind of a not chronic inflammation, but a hit of inflammation. When we get around to talking about hyperthermia, I can tell you that there’s some evidence that hyperthermia does that, exercise does that. You know, exercise acutely activates certain types of pathways we think about as being inflammatory. So I think in the next 10 years, what we’re going to find out is that, in fact, the immune system is probably involved in every case of depression, but that the pattern is going to be subtler and more complex than something just saying that depression is associated with increased inflammation. That’s probably not going to turn out to be true.
Programmed cell death. Apoptosis is a type of cellular self-destruct mechanism that rids the body of damaged or aged cells. Unlike necrosis, a process in which cells that die as a result of acute injury swell and burst, spilling their contents over their neighbors and causing a potentially damaging inflammatory response, a cell that undergoes apoptosis dies in a neat and orderly fashion – shrinking and condensing, without damaging its neighbors. The process of apoptosis is often blocked or impaired in cancer cells. (May be pronounced “AY-pop-TOE-sis” OR “AP-oh-TOE-sis”.)
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A broad category of small proteins (~5-20 kDa) that are important in cell signaling. Cytokines are short-lived proteins that are released by cells to regulate the function of other cells. Sources of cytokines include macrophages, B lymphocytes, mast cells, endothelial cells, fibroblasts, and various stromal cells. Types of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factor.
A general term referring to cognitive decline that interferes with normal daily living. Dementia commonly occurs in older age and is characterized by progressive loss of memory, executive function, and reasoning. Approximately 70 percent of all dementia cases are due to Alzheimer’s disease.
A mood disorder characterized by profound sadness, fatigue, altered sleep and appetite, as well as feelings of guilt or low self-worth. Depression is often accompanied by perturbations in metabolic, hormonal, and immune function. A critical element in the pathophysiology of depression is inflammation. As a result, elevated biomarkers of inflammation, including the proinflammatory cytokines interleukin-6 and tumor necrosis factor-alpha, are commonly observed in depressed people. Although selective serotonin reuptake inhibitors and cognitive behavioral therapy typically form the first line of treatment for people who have depression, several non-pharmacological adjunct therapies have demonstrated effectiveness in modulating depressive symptoms, including exercise, dietary modification (especially interventions that capitalize on circadian rhythms), meditation, sauna use, and light therapy, among others.
A neurotransmitter best known for its role in motor, motivation, and pleasure control. Dopamine also functions as a paracrine (cell-to-cell) hormone in other parts of the body. It is derived from tyrosine and is the precursor to norepinephrine and epinephrine. Some evidence suggests that dopamine may also be involved in pain modulation.
A type of toxin released when bacteria die. Endotoxins can leak through the intestinal wall and pass directly into the bloodstream. The most common endotoxin is lipopolysaccharide (LPS), a major component of the cell membrane of gram-negative bacteria. If LPS leaks into the bloodstream, it can trigger an acute inflammatory reaction. LPS has been linked with a number of chronic diseases, including Alzheimer’s disease, inflammatory bowel disease (Crohn’s disease or ulcerative colitis), cardiovascular disease, diabetes, obesity, autoimmune disorders (celiac disease, multiple sclerosis, and type 1 diabetes), and psychiatric disorders (anxiety and depression).
A broad class of supportive cells in the central nervous system. Glial cells surround and provide support for and insulation between neurons. Unlike neurons, glial cells do not conduct electrical impulses. Glial cells are the most abundant cell types in the central nervous system, outnumbering neurons by a ratio of roughly 3 to 1. They are generally smaller than neurons, and they lack axons and dendrites. Types of glial cells include oligodendrocytes, astrocytes, ependymal cells, Schwann cells, microglia, and satellite cells.
Biological responses to low-dose exposures to toxins or other stressors such as exercise, heat, cold, fasting, and xenohormetics. Hormetic responses are generally favorable and elicit a wide array of protective mechanisms. Examples of xenohormetic substances include plant polyphenols – molecules that plants produce in response to stress. Some evidence suggests plant polyphenols may have longevity-conferring effects when consumed in the diet.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A type of white blood cell. Macrophages engulf and digest cellular debris, foreign substances, microbes, cancer cells, and oxidized LDL in a process called phagocytosis. After phagocytizing oxidized LDL, macrophages are referred to as foam cells.
In general, anything that can produce disease. Typically, the term is used to describe an infectious agent such as a virus, bacterium, prion, fungus, or other microorganism.
One of the most metabolically active regions in the brain. The posterior cingulate likely integrates information in the brain, but no scientific consensus currently exists regarding its role in cognitive function. The posterior cingulate is activated during self-related thinking and deactivated during meditation. Disorders associated with the posterior cingulate include depression, Alzheimer's disease, autism, attention deficit-hyperactivity disorder, traumatic brain injury, and schizophrenia.
A pathogen that has been shown to alter the behavior of infected rodents in ways that increase their chances of being preyed upon by felids. Support for this "manipulation hypothesis" stems from studies showing T. gondii-infected rats have a decreased aversion to cat urine. Because cats are the only hosts within which T. gondii can sexually reproduce to complete and begin its lifecycle (a "definitive host"), such behavioral manipulations are thought to be evolutionary adaptations that increase the parasite's reproductive success. Though humans and other mammals are not definitive hosts of T. gondii, infection is thought to be widespread with estimates in countries like France as high as 84%.
Pertaining to sustenance. Trophic factors are critical for brain development and may help treat or prevent brain injuries and disorders. Examples include modulators of growth and maintenance, such as insulin and nerve growth factor.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.