Contents
Sirtuins are highly conserved enzymes that play key roles in healthspan and longevity in multiple organisms. They are linked to the regulation of a variety of metabolic processes, including the release of insulin, mobilization of lipids, response to stress, and modulation of lifespan.[1] [2] Sirtuins have been implicated in the pathophysiology of metabolic disorders such as type 2 diabetes; neurological disorders such as Parkinson’s disease; and many other conditions related to aging.[3] [4] [5]
Sirtuins respond to physiological changes in energy levels, thereby regulating energy homeostasis and health. There are seven known sirtuins in mammals (SIRT1 to SIRT7) with varied functions and cellular localizations. The most extensively studied sirtuin, SIRT1, has been shown to modulate histones, transcription factors, and DNA repair proteins – all genome level processes thought to play important roles in aging. Broad loss of sirtuin activity is typically observed with aging, and many animal models demonstrate that decreased SIRT1 activity may promote the pathogenesis of both cardiovascular and neurological diseases.[6] [7] Conversely, increased sirtuin activity delays the processes of aging and mediates many of the beneficial effects seen with caloric restriction, one of the few successful aging interventions that demonstrates lifespan extension across virtually all major scientific models of aging, ranging from single celled organisms to primates.[8]
Sirtuin regulation as a cellular sensor
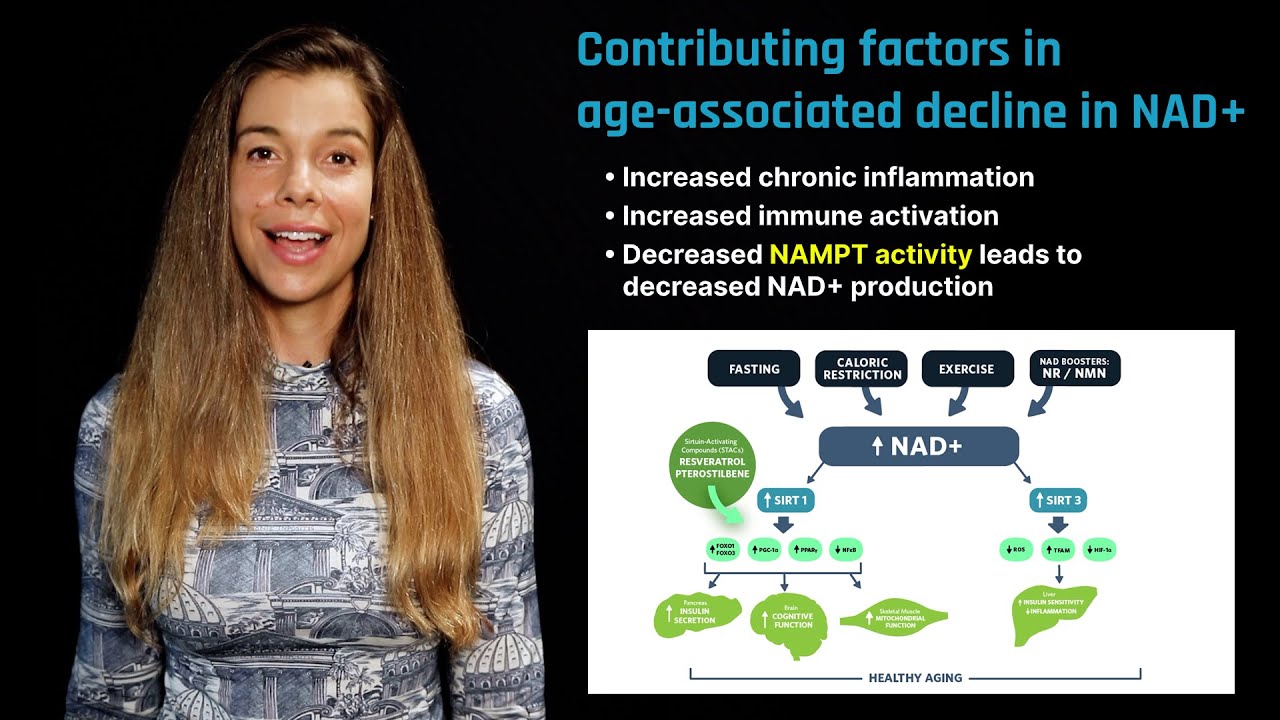
Fasting, caloric restriction, exercise, and NAD+ boosters nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) all increase NAD+, leading to activation of SIRT1 and SIRT3 and resulting in a myriad of tissue-specific effects that improve healthspan. Sirtuin-activating compounds such as resveratrol and pterostilbene directly activate SIRT1.
Sirtuin activity is regulated by dynamic changes in cellular levels of nicotinamide adenine dinucleotide, or NAD+, a coenzyme that is required for the production of energy in cells. Manipulation of cellular levels of NAD+ offers a promising strategy for aging interventions. For example, supplementation with nicotinamide riboside, a form of vitamin B3 (also known as niacin) may increase NAD+ levels within the cells of some tissues, stimulating some of the cellular changes associated with the benefits of caloric restriction.
Sirtuins utilize NAD+ to remove specific chemical structures called acetyl groups – a process called deacetylation – from cellular proteins to control transcriptional regulation, energy metabolism, circadian rhythms, DNA repair, and cell survival. When cellular energy levels are low, such as during exercising, fasting, or caloric restriction, the ratio of NAD+ to its reduced form, NADH, increases, thereby serving as a "sensor" to switch on sirtuin expression and subsequent activity.
Caloric restriction, in particular, has been shown to increase lifespan in mice and decrease age-related disease in humans, mediated through the increased expression of Sirt1, Sirt3, and Sirt5 (in mice) and SIRT1 (in humans). [9] [10] [11] Transgenic mice that overexpress Sirt1 display phenotypes that are similar to mice fed a calorie-restricted diet, including reduced blood cholesterol and insulin levels, improved glucose tolerance, and decreased body weights, compared to wild-type mice. Conversely, mice that lack the Sirt1 gene have a shorter lifespan than wild-type animals, and the lifespan-enhancing benefits of a calorie-restricted diet are negated.[12] [13] [14] Aging and states of excess cellular energy, however, such as obesity or eating a high-fat diet, result in a loss of sirtuin activity in both mice and humans, possibly providing a mechanism for the links between excess energy intake and metabolic dysregulation.[15] [16] [17]
Learn more about the role of NAD+ in aging in this episode featuring Dr. Eric Verdin.
Sirtuin-activating compounds (SACs)
Some naturally occurring and synthetic compounds, called sirtuin-activating compounds, mimic conditions of low cellular energy. Sirtuin activating compounds bind to sirtuins, altering their affinity for NAD+ and their protein substrates, thereby increasing sirtuins' activity. A growing body of evidence suggests that sirtuin-activating compounds show promise as therapeutic approaches for treating metabolic dysfunction and age-related diseases.[6]
For example, resveratrol, a polyphenolic dietary compound found in red grapes, is a potent sirtuin activating compound that demonstrates protective effects against type 2 diabetes, cardiomyopathy, and cancer.[18] [19] [20]
"Resveratrol, a polyphenolic dietary compound found in red grapes, is a potent sirtuin activating compound that demonstrates protective effects against type 2 diabetes, cardiomyopathy, and cancer." Click To Tweet
Learn more about depression in this overview article.
Pterostilbene, a polyphenolic compound that is related to resveratrol, is found in blueberries, cranberries, and almonds. Like resveratrol, pterostilbene is a sirtuin activating compound. However, pterostilbene's molecular structure makes it more bioavailable – as much four times more pterostilbene is taken up into the bloodstream as resveratrol upon ingestion.[21] Some studies suggest that pterostilbene is more potent than resveratrol when it comes to improving brain function, averting some types of cancer, and preventing heart disease.[22] It also demonstrates greater potency than resveratrol in improving cognitive function in progeria aged mice.[23] However, in a randomized, double-blind, and placebo-controlled study involving 80 people who had high cholesterol, participants who took a pterostilbene supplement twice daily for six to eight weeks exhibited increased LDL levels.[24]
Finally, metformin, a drug commonly used to treat diabetes, demonstrates potent sirtuin activating capacity. Like resveratrol and pterostilbene, metformin binds to specific sites on SIRT1. In turn, it improves the enzymatic efficiency of SIRT1 when cellular levels of NAD+ are low.[25] Learn more about metformin in this overview article.
Physiological roles of sirtuins
Sirtuins play many roles across a broad spectrum of disease states and biological processes, including those that have the capacity to modulate the onset of age-related diseases such as neurodegenerative disorders and metabolic dysfunction.
Sirtuins as an enhancer of fuel utilization
Under conditions of energy excess, fats are stored as fatty acids within specialized cells called white adipocytes, and glucose is stored as glycogen in the liver and muscles. When the body is stressed due to low energy levels, it undergoes a metabolic shift that facilitates the use of stored nutrients via the processes of fatty acid oxidation and glucose metabolism.
Fatty acid oxidation
SIRT1 activity promotes fat mobilization from white adipocytes and inhibits adipogenesis (the process by which adipocytes mature) by suppressing activity of the transcription factor PPAR-gamma.[26] It also promotes fatty acid oxidation in skeletal muscle while suppressing its synthesis in the liver.[27] Overexpression of SIRT1 protects mice from hepatic steatosis, a condition characterized by fat accumulation in the liver. Conversely, deletion of the SIRT1 gene promotes hepatic steatosis via increased fatty acid synthesis.[28] [29] [30]
Mitochondria are critical to the utilization of fatty acids. Mitochondrial biogenesis, the process by which new mitochondria are produced in cells, is regulated by the transcription factor PGC-1α. SIRT1 promotes the synthesis of PGC1-α to induce mitochondrial biogenesis in several cell types.[31] [32]
Glucose metabolism
SIRT1 activity also stimulates the transcription of proteins involved in gluconeogenesis – the production of glucose from non-carbohydrate precursors – by activating FOXO1.[33] [34] However, when energy levels are high, SIRT1 activity inhibits glucose metabolism (glycolysis) by suppressing the transcription of proteins involved in the glycolytic process.[31] SIRT1 also regulates insulin secretion from the pancreas, a process tightly coupled to blood glucose levels, by suppressing the activity of uncoupling protein 2, a protein that participates in energy production in the cell, leading to an increase of pancreatic ATP and, subsequently, insulin release.[35]
Sirtuins as modulators of inflammation
Chronic local inflammation in tissues such as the liver and adipose tissue is a hallmark of metabolic syndrome, a collection of disorders that increases a person's risk of developing cardiovascular disease, diabetes, fatty liver, and several types of cancer.[36] [37] SIRT1 has been shown to modulate inflammation by suppressing the activity of NF-κB, a family of inducible transcription factors that regulates genes involved in various immune and inflammatory responses.[38] However, deleting the SIRT1 gene in mouse macrophages (a type of white blood cell of the immune system) leads to increased inflammatory gene expression within the cells.[39]
Sirtuins as mediators of autophagy
Autophagy is a critical cellular defense mechanism that facilitates the removal of damaged or dysfunctional cellular components. It is a key player in the regulation of the aging process. Multiple studies demonstrate that SIRT1 mediates autophagy induction by interacting with components of the autophagy machinery such as FOXO1 and FOXO3a.[40] [41]
Learn more about autophagy in this overview article.
Circadian regulation
The circadian system is highly intertwined with an organism's metabolism to optimize performance over a 24-hour cycle. Sirtuins have been shown to regulate the circadian clocks in both brain and peripheral tissues.
In the suprachiasmatic nucleus of the brain, SIRT1 is expressed in a circadian manner and can regulate the expression of core clock genes CLOCK and BMAL1. SIRT1 has been shown to directly bind to CLOCK/BMAL1 to promote the deacetylation of BMAL1, thereby influencing its activity.[42] Furthermore, SIRT1 can also promote the degradation of PER2, a protein that regulates circadian rhythms of metabolism, locomotor activity, and behavior.[43]
In the liver, SIRT1 modulates the circadian expression of acetyl-CoA synthetase 1, an enzyme that controls fatty acid elongation by producing acetyl-CoA.[44] SIRT6 has also been shown to interact with CLOCK/BMAL and can control the cyclic expression of the transcription factor SREBP-1, which is important for regulating aspects of lipid metabolism such as fatty acid synthesis and storage.[45]
Some evidence suggests that sirtuin activating compounds can restore the circadian rhythmic dysregulation of lipid metabolism induced by high-fat diet feeding in mice by acting as a zeitgeber signal – an external cue that has the capacity to influence the body's internal clocks.[46] Preliminary data in liver cells have shown that insulin resistance due to circadian dysregulation can be recovered through the activation of SIRT1 by resveratrol. [47]
Neuroprotection and slowed cognitive aging
SIRT1 is ubiquitous in the mammalian brain; however, levels decrease with aging, high-fat diet eating, and neuropathological conditions.[48] [49] In animal models, deletion of the SIRT1 gene impairs cognitive functions such as short and long-term associative memory, as well as spatial learning.[50] [51] Furthermore, in rodent models of Alzheimer's disease, SIRT1 activation with resveratrol or via genetic overexpression reduces amyloid plaque formation and consequently delays neurodegeneration and cognitive decline. [52] [53] [54] [55]
In humans, two separate studies demonstrated that SIRT1 activity is reduced in post-mortem brain tissue obtained from people who had Alzheimer's disease and Parkinson's disease.[56] [57] In addition, a randomized, placebo-controlled, double-blind trial involving people diagnosed with mild-to-moderate dementia due to Alzheimer's disease found that participants who took a resveratrol supplement twice a day (up to 1 gram) for one year exhibited reduced levels of MMP9, a neurological inflammatory marker associated with increased neurodegeneration and inflammation. The participants also showed improvements in mental status and their ability to carry out activities of daily living.[58] While these studies support the idea of SIRT1 influencing the pathophysiology of Alzheimer's disease, more clinical studies are necessary to investigate whether targeting SIRT1 will display positive outcomes in patients with neurological diseases.
Cancer
An abundance of data indicates that sirtuins act as both tumor-suppressors and tumor-promoters in various types of cancer. For example, whereas overexpression of SIRT1 suppresses tumor growth in colon cancer, its upregulation promotes tumor growth in gastric cancer.[59] [60] One or both copies of the SIRT3 gene is absent in 20 percent of all cancers, a figure that is doubled in breast and ovarian cancers.[61] Genetic observations like these don't necessarily act as a signpost for expected whole-organism effects of sirtuin activating compounds, for example. However, they do provide a more complete picture of the way in which cancer can often hijack otherwise beneficial and life-sustaining mechanisms toward its own selfish objectives. Future animal trials and studies of human tissue will likely elucidate the roles that sirtuins play in various types of cancer.
Conclusion
Sirtuins are crucial cellular proteins that hold sway over a broad range of physiological processes, including circadian regulation, anti-inflammatory activity, metabolic adaptations, and neurological protection. As such, they present a promising therapeutic strategy to ameliorate age-related diseases and extend healthspan. The focus of current sirtuin-related research is centered on the use of NAD+ supplementation or sirtuin-activating compounds such as resveratrol to boost sirtuin activity to revert the disease process and promote healthy aging.
- ^ Chang, Hung-Chun; Guarente, Leonard (2014). SIRT1 And Other Sirtuins In Metabolism Trends In Endocrinology & Metabolism 25, 3.
- ^ Lomb, David J.; Laurent, Gaëlle; Haigis, Marcia C. (2010). Sirtuins Regulate Key Aspects Of Lipid Metabolism Biochimica Et Biophysica Acta (BBA) - Proteins And Proteomics 1804, 8.
- ^ Kitada, Munehiro; Ogura, Yoshio; Monno, Itaru; Koya, Daisuke (2019). Sirtuins And Type 2 Diabetes: Role In Inflammation, Oxidative Stress, And Mitochondrial Function Frontiers In Endocrinology 10, .
- ^ Tang, Bor Luen (2016). Sirtuins As Modifiers Of Parkinson's Disease Pathology Journal Of Neuroscience Research 95, 4.
- ^ Sack, M. N.; Finkel, T. (2012). Mitochondrial Metabolism, Sirtuins, And Aging Cold Spring Harbor Perspectives In Biology 4, 12.
- ^ a b 30355082
- ^ Xu, Jing; Jackson, Charlie W.; Khoury, Nathalie; Escobar, Iris; Perez-Pinzon, Miguel A. (2018). Brain SIRT1 Mediates Metabolic Homeostasis And Neuroprotection Frontiers In Endocrinology 9, .
- ^ De Cabo, Rafael; Colman, Ricki J; Mattison, Julie A.; Beasley, T. Mark; Allison, David B.; Kemnitz, Joseph W., et al. (2017). Caloric Restriction Improves Health And Survival Of Rhesus Monkeys Nature Communications 8, 1.
- ^ Sinclair, D A; Kessler, Benedikt; De Cabo, Rafael; Wall, Nathan R; Cohen, Haim Y.; Miller, Christine, et al. (2004). Calorie Restriction Promotes Mammalian Cell Survival By Inducing The SIRT1 Deacetylase Science 305, 5682.
- ^ 17923681
- ^ Nakagawa, Takashi; Haigis, Marcia C.; Guarente, Leonard; Lomb, David J. (2009). SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 And Regulates The Urea Cycle Cell 137, 3.
- ^ Li, Ying; Xu, Wei; McBurney, Michael W.; Longo, Valter D. (2008). SirT1 Inhibition Reduces IGF-I/IRS-2/Ras/ERK1/2 Signaling And Protects Neurons Cell Metabolism 8, 1.
- ^ Harper, Mary-Ellen; Crawford, Sean; Boily, Gino; Seifert, Erin L.; Bevilacqua, Lisa; He, Xiao Hong, et al. (2008). SirT1 Regulates Energy Metabolism And Response To Caloric Restriction In Mice Plos One 3, 3.
- ^ De Cabo, Rafael; Wei, Min; Mercken, Evi M.; Hu, Jia; Krzysik-Walker, Susan; Li, Ying, et al. (2013). SIRT1 But Not Its Increased Expression Is Essential For Lifespan Extension In Caloric-Restricted Mice Aging Cell 13, 1.
- ^ Chalkiadaki, Angeliki; Guarente, Leonard (2012). High-Fat Diet Triggers Inflammation-Induced Cleavage Of SIRT1 In Adipose Tissue To Promote Metabolic Dysfunction Cell Metabolism 16, 2.
- ^ Pedersen, S B; Richelsen, Bjørn; Ølholm, J; Paulsen, S K; Bennetzen, M F (2008). Low Sirt1 Expression, Which Is Upregulated By Fasting, In Human Adipose Tissue From Obese Women International Journal Of Obesity 32, 8.
- ^ Padoin, Alexandre Vontobel; Rohden, Francieli; Costa, Cíntia Dos Santos; Hammes, Thais Ortiz; Margis, Rogério; Bortolotto, Josiane Woutheres, et al. (2009). SIRT1 Transcription Is Decreased In Visceral Adipose Tissue Of Morbidly Obese Patients With Severe Hepatic Steatosis Obesity Surgery 20, 5.
- ^ Cao, Ming-Ming; Lu, Xi; Liu, Guo-Dong; Su, Ying; Li, Yan-Bo; Zhou, Jin (2017). Resveratrol Attenuates Type 2 Diabetes Mellitus By Mediating Mitochondrial Biogenesis And Lipid Metabolism Via Sirtuin Type 1 Experimental And Therapeutic Medicine , .
- ^ Han, Dong; Cao, Feng; Ma, Sai; Feng, Jing; Wang, Yabin; Zhang, Ran, et al. (2017). SIRT1 Activation By Resveratrol Alleviates Cardiac Dysfunction Via Mitochondrial Regulation In Diabetic Cardiomyopathy Mice Oxidative Medicine And Cellular Longevity 2017, .
- ^ Huang, Kuo-How; Yang, Ting-Hua; Huang, Kuo-Yuan; Tang, Chih-Hsin; Yang, Rong-Sen; Wang, Ching-Chia, et al. (2017). Induction Of Sirtuin-1 Signaling By Resveratrol Induces Human Chondrosarcoma Cell Apoptosis And Exhibits Antitumor Activity Scientific Reports 7, 1.
- ^ Kapetanovic, Izet M.; Muzzio, Miguel; Huang, Zhihua; Thompson, Thomas N.; McCormick, David L. (2010). Pharmacokinetics, Oral Bioavailability, And Metabolic Profile Of Resveratrol And Its Dimethylether Analog, Pterostilbene, In Rats Cancer Chemotherapy And Pharmacology 68, 3.
- ^ Lin, Chi-Chen; Li, Shiming; Li, Yi-Rong (2017). Effect Of Resveratrol And Pterostilbene On Aging And Longevity BioFactors 44, 1.
- ^ Shukitt-Hale, Barbara; Casadesus, Gemma; Camins, Antoni; Chang, Jaewon; Rimando, Agnes; Pallas, Merce, et al. (2012). Low-dose Pterostilbene, But Not Resveratrol, Is A Potent Neuromodulator In Aging And Alzheimer's Disease Neurobiology Of Aging 33, 9.
- ^ Riche, Daniel M.; Riche, Krista D.; Blackshear, Chad T.; McEwen, Corey L.; Sherman, Justin J.; Wofford, Marion R., et al. (2014). Pterostilbene On Metabolic Parameters: A Randomized, Double-Blind, And Placebo-Controlled Trial Evidence-Based Complementary And Alternative Medicine 2014, .
- ^ Brunet, Joan; Bosch-Barrera, Joaquim; Menendez, Javier A; Fernández-Arroyo, Salvador; Sanchez-Martinez, Melchor; Verdura, Sara, et al. (2018). Metformin Is A Direct SIRT1-Activating Compound: Computational Modeling And Experimental Validation Frontiers In Endocrinology 9, .
- ^ De Oliveira, Rita Machado; Picard, Frédéric; Kurtev, Martin; Chung, Namjin; Topark-Ngarm, Acharawan; Senawong, Thanaset, et al. (2004). Sirt1 Promotes Fat Mobilization In White Adipocytes By Repressing PPAR-γ Nature 429, 6993.
- ^ Houten, Sander M; Canto, Carles; Milne, Jill C.; Lambert, Philip D.; Mataki, Chikage; Elliott, Peter J., et al. (2008). Specific SIRT1 Activation Mimics Low Energy Levels And Protects Against Diet-Induced Metabolic Disorders By Enhancing Fat Oxidation Cell Metabolism 8, 5.
- ^ 20817729
- ^ Velasco-Miguel, Susana; Tschöp, Matthias H.; Serrano, Manuel; Pfluger, Paul; Herranz, Daniel (2008). Sirt1 Protects Against High-Fat Diet-Induced Metabolic Damage Proceedings Of The National Academy Of Sciences 105, 28.
- ^ Li, Xiaoling; Purushotham, Aparna; Schug, Thaddeus T.; Xu, Qing; Surapureddi, Sailesh; Guo, Xiumei (2009). Hepatocyte-Specific Deletion Of SIRT1 Alters Fatty Acid Metabolism And Results In Hepatic Steatosis And Inflammation Cell Metabolism 9, 4.
- ^ a b Rodgers, Joseph T; Lerin, Carlos; Haas, Wilhelm; Gygi, Steven P.; Spiegelman, Bruce M.; Puigserver, Pere (2005). Nutrient Control Of Glucose Homeostasis Through A Complex Of PGC-1α And SIRT1 Nature 434, 7029.
- ^ Rodgers, Joseph T; Lerin, Carles; Gerhart-Hines, Zachary; Puigserver, Pere (2007). Metabolic Adaptations Through The PGC-1α And SIRT1 Pathways FEBS Letters 582, 1.
- ^ Dentin, Renaud; Chen, Danica; Hedrick, Susan; Ravnskjaer, Kim; Milne, Jill; Meyers, David J., et al. (2008). A Fasting Inducible Switch Modulates Gluconeogenesis Via Activator/Coactivator Exchange Nature 456, 7219.
- ^ 10.1074/jbc.m412357200
- ^ Lemieux, Madeleine; Apfeld, Javier; Bordone, Laura; Motta, Maria Carla; Picard, Frederic; Robinson, Ashley, et al. (2005). Sirt1 Regulates Insulin Secretion By Repressing UCP2 In Pancreatic Β Cells PLOS Biology 4, 2.
- ^ Grundy, Scott M. (2016). Metabolic Syndrome Update Trends In Cardiovascular Medicine 26, 4.
- ^ Micucci, Carla; Valli, Debora; Matacchione, Giulia; Catalano, Alfonso (2016). Current Perspectives Between Metabolic Syndrome And Cancer Oncotarget 7, 25.
- ^ Jones, David; Yeung, Fan; Hoberg, Jamie E; Ramsey, Catherine S; Keller, Michael D; Frye, Roy A, et al. (2004). Modulation Of NF-κB-dependent Transcription And Cell Survival By The SIRT1 Deacetylase The EMBO Journal 23, 12.
- ^ Schenk, Simon; Yoshizaki, Takeshi; Imamura, Takeshi; Babendure, Jennie L.; Sonoda, Noriyuki; Bae, Eun Ju, et al. (2010). SIRT1 Inhibits Inflammatory Pathways In Macrophages And Modulates Insulin Sensitivity American Journal Of Physiology-Endocrinology And Metabolism 298, 3.
- ^ 20947830
- ^ 20335657
- ^ Chang, Hung-Chun; Guarente, Leonard (2013). SIRT1 Mediates Central Circadian Control In The SCN By A Mechanism That Decays With Aging Cell 153, 7.
- ^ Gatfield, David; Asher, Gad; Stratmann, Markus; Reinke, Hans; Dibner, Charna; Kreppel, Florian, et al. (2008). SIRT1 Regulates Circadian Clock Gene Expression Through PER2 Deacetylation Cell 134, 2.
- ^ 24425865
- ^ Sassone-Corsi, Paolo; Roqueta-Rivera, Manuel; Sebastian, Carlos; Cervantes, Marlene; Masri, Selma; Rigor, Paul, et al. (2014). Partitioning Circadian Transcription By SIRT6 Leads To Segregated Control Of Cellular Metabolism Cell 158, 3.
- ^ Sun, Linjie; Wang, Yan; Song, Yu; Cheng, Xiang-Rong; Xia, Shufang; Rahman, Md Ramim Tanver, et al. (2015). Resveratrol Restores The Circadian Rhythmic Disorder Of Lipid Metabolism Induced By High-Fat Diet In Mice Biochemical And Biophysical Research Communications 458, 1.
- ^ Zhang, Yi; Zhang, Fang; Xia, Yulei; Liu, Jun; Huang, Rui; Wang, Yuangao, et al. (2014). CLOCK/BMAL1 Regulates Circadian Change Of Mouse Hepatic Insulin Sensitivity By SIRT1 Hepatology 59, 6.
- ^ Wu, Aiguo; Ying, Zhe; Gomez-Pinilla, Fernando (2006). Oxidative Stress Modulates Sir2α In Rat Hippocampus And Cerebral Cortex European Journal Of Neuroscience 23, 10.
- ^ Quintas, Ana; De Solís, Alain J.; Díez-Guerra, F. Javier; Carrascosa, José M.; Bogónez, Elena (2012). Age-associated Decrease Of SIRT1 Expression In Rat Hippocampus Experimental Gerontology 47, 2.
- ^ 20660252
- ^ Mao, Yingwei; Guan, Ji-Song; Gräff, Johannes; Pan, Ling; Mak, Gloria; Kim, Dohoon, et al. (2010). A Novel Pathway Regulates Memory And Plasticity Via SIRT1 And miR-134 Nature 466, 7310.
- ^ 20080969
- ^ Karuppagounder, Saravanan S.; Pinto, John T.; Xu, Hui; Chen, Huan-Lian; Beal, M. Flint; Gibson, Gary E. (2009). Dietary Supplementation With Resveratrol Reduces Plaque Pathology In A Transgenic Model Of Alzheimer's Disease Neurochemistry International 54, 2.
- ^ Sinclair, D A; Rodgers, Joseph T; Sui, Guangchao; Delalle, Ivana; Baur, Joseph A; Kim, Dohoon, et al. (2007). SIRT1 Deacetylase Protects Against Neurodegeneration In Models For Alzheimer's Disease And Amyotrophic Lateral Sclerosis The EMBO Journal 26, 13.
- ^ 10.1074/jbc.m602909200
- ^ 19104446
- ^ Morris, Chris; Singh, Preeti; Hanson, Peter S. (2017). SIRT1 Ameliorates Oxidative Stress Induced Neural Cell Death And Is Down-Regulated In Parkinson’s Disease BMC Neuroscience 18, 1.
- ^ Turner, Scott; Huang, Xu; Moussa, Charbel; Hebron, Michaeline; Ahn, Jaeil; Rissman, Robert A., et al. (2017). Resveratrol Regulates Neuro-Inflammation And Induces Adaptive Immunity In Alzheimer’s Disease Journal Of Neuroinflammation 14, 1.
- ^ Sinclair, D A; De Cabo, Rafael; Ogino, Shuji; Firestein, Ron; Blander, Gil; Michan, Shaday, et al. (2008). The SIRT1 Deacetylase Suppresses Intestinal Tumorigenesis And Colon Cancer Growth Plos One 3, 4.
- ^ Li, Hai-Jun; Che, Xiang-Ming; Zhao, Wei; He, Shi-Cai; Zhang, Zheng-Liang; Chen, Rui, et al. (2013). Diet-induced Obesity Promotes Murine Gastric Cancer Growth Through A Nampt/Sirt1/C-Myc Positive Feedback Loop Oncology Reports 30, 5.
- ^ 22589271
Topics related to Aging
-
Fasting
Fasting – the voluntary abstinence from food and drink – is an ancient practice now widely appreciated for its beneficial effects on healthspan.
-
FOXO
FOXO proteins are transcriptional regulators that play an important role in healthy aging. Some FOXO genes may increase lifespan.
-
Time-restricted eating
Time-restricted eating is a form of daily fasting wherein a person eats only during a limited time window, typically 8- to 12-hours.
-
Resveratrol
Resveratrol is a polyphenolic compound produced in plants that demonstrates anti-inflammatory and anti-aging properties in humans.
-
NAD+
NAD+ is a cofactor that plays an essential role in metabolism, DNA repair, and immunity. Its depletion accelerates aging.
-
Nicotinamide riboside
Nicotinamide riboside is a precursor of NAD+, a coenzyme necessary for energy production and cellular repair. It is available from food and supplements.