#36 Judith Campisi, Ph.D., on Cellular Senescence, Mitochondrial Dysfunction, Cancer & Aging
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
Judith Campisi, PhD, is a professor of biogerontology at the Buck Institute for Research on Aging. She is an expert in understanding why age is the greatest risk factor for developing a wide array of diseases, from neurodegeneration to cancer.
Dr. Campisi is widely known for her groundbreaking research in the field of cellular senescence – a state of irreversible growth arrest in cells – and its role in tumor suppression and aging. Her research merges the varied genetic, environmental, and evolutionary forces that drive aging and age-related diseases, and identifies modifiable pathways that can mitigate basic aging processes. Dr. Campisi has contributed to several discoveries that continue to challenge and alter current thinking about the science of aging. She is perhaps best known for her research into understanding what happens when cells that are damaged (especially those that undergo mitochondrial dysfunction-associated senescence, or MiDAS) acquire the senescence-associated secretory phenotype, or SASP, which is a characteristic secretory pattern that senescent cells adapt and has the ability to promote tumor progression.
Dr. Campisi earned her PhD in biochemistry from the State University New York at Stony Brook and completed postdoctoral training at the Harvard Medical School. She is an advisory board member of the SENS Research Foundation and an advisor at the Lifeboat Foundation. She is co-editor in chief of the Aging Journal.
In this episode, Dr. Campisi tells us about the role of cellular senescence in the aging process and cancer, what causes senescence, and how strategies that address senescence may ultimately inform ways to improve lifespan, or at the very least, healthspan, in humans. These strategies might include lifestyle interventions that clear senescent cells, such as fasting and exercise; pharmacological treatments that alter the phenotype of senescent cells by suppressing their inflammatory qualities, such as rapamycin; and the use of senolytic drugs that kill senescent cells.
Cellular senescence as both protector and instigator.
"Anything that persistent damage to the genome will drive cells into senescence. It makes sense because that puts you at risk for mutations. Mutation puts you at risk for cancer, so the cells want to shut that damaged cell down."- Dr. Judith Campisi Click To Tweet
As our cells accumulate damage, which naturally happens as we age (even as a consequence of energy-generating processes and immune cell activation), there are only so many outcomes that we can expect. The first possibility is that the cells can die. The second is that they can become senescent – where they stop dividing, but stay alive, all-the-while secreting molecules that influence surrounding tissue. Or, the worst of all possible outcomes, the cells can go off the rails and become malignant.
Senescence as a response to stress.
Our bodies’ cells are subject to myriad stressors, from dietary indiscretions to environmental toxins to the metabolic byproducts of day-to-day living. Over time, stressed cells lose their ability to divide. These are senescent cells, and over time they begin to accumulate. Very few senescent cells are found in young people, but after about the fourth or fifth decade of life, the number increases rapidly.
In this way, the senescence stress response is like a double-edged sword, with both favorable and unfavorable outcomes. Favorable outcomes include, for example, avoiding division of an already damaged cell that could otherwise be on its own way to malignancy. Unfavorable outcomes include, for example, changing the behavior of our tissues and promoting a tissue and cellular milieu that promotes a problem known in the aging field as inflammaging. Recent research suggests that suppression of chronic, low-grade inflammation may be one of the most important factors in successful longevity that actually increases in importance with advancing age.
Mitigating the effects of senescence as a potential boon to aging.
"Exercise is probably the single most important intervention that cuts across multiple diseases."- Dr. Judith Campisi Click To Tweet
What's interesting is that, while accumulating senescent cells is inevitable, there are varying strategies for tackling senescence, and this is of great interest to the field of aging. There are ways to clear out senescent cells with drugs or even dietary and lifestyle interventions. Some of these strategies include periodic fasting, which serves as a sort of “housekeeping” function – ridding the body of senescent cells from time to time, and exercise, which acts as a hormetic factor – a low-level stressor that primes your body to respond more effectively to larger ones and may also be especially suited to tuning up the mitochondria, the dysfunction of which can promote cellular senescence.
There are also ways to influence what sort of molecules senescent cells produce, possibly limiting the inflammatory ones, even without killing them. The outcome of these interventions is an extension of healthspan – the years of a person’s life spent free of disease. Although we all eventually succumb to aging and death, we might be able to live healthier longer.
Finally, of course, we can also try to prevent them, which poses the question of what causes them in the first place. As we'll learn from Dr. Campisi, senescent cells exhibit more than one so-called phenotype, and they arrive at these different cellular phenotypes as a consequence of different types of cellular stress or dysfunction.
Some of the factors contributing to these variable phenotypes include persistent damage to our DNA and mitochondrial dysfunction, driven by an altered NAD:NADH ratio, the latter of which has recently been shown to be improved by certain dietary supplements like nicotinamide riboside. Notably, NAD+ is a substrate for an enzyme called PARP, which repairs single-strand breaks in DNA. DNA damage is an aging-associated burden that accumulates, at least partially, due to the failing capacity of our own enzymes to keep up with damage.
The secretions of senescent cells can have profound effects on stem cell proliferation and function. Understanding how cells become senescent and how we might prevent or slow those processes is critical to improving our overall health and longevity.
Learn more about Dr. Judy Campisi
- Judith Campisi - Buck Profile
- Judith Campisi - USC Leonard Davis School of Gerontology
- Wikipedia Profile on Judith Campisi, Ph.D.
- List of publications
People mentioned
-
Antagonistic pleiotropy provides an evolutionary theory of aging
-
Fundamental molecular processes of aging
-
Rates of aging and inflammaging differ between people
-
The innate immune response can be both friend and foe
-
Inflammatory immune cells accumulate in tissues as we age
-
The destructive feedback loop of immune activation and cellular damage
-
Gut permeability and inflammation increases with age
-
Why cellular senescence exists
-
Senescent cells drive an inflammatory feedback loop, increasing their numbers
-
The epithelial to mesenchymal transition enhances cancer metastasis and progression
-
Why diseases of aging begin to crop up around age 50 to 60
-
Senescent cells drive the development of cancer in older people
-
The clearance of senescent cells extends lifespan in mice
-
Why it might be a bad idea to kill off senescent cells
-
Removing senscent cells in pulses is the optimal strategy
-
How to compress mortality and increase longevity
-
Why supporting brain cells called astrocytes give rise to cancer
-
How mitochondrial dysfunction causes senescence
-
Senescent cells have phenotypes readily identified by their secretory profile
-
How the immune system changes function over time
-
Why senescent cells accumulate with age
-
The effects of prolonged fasting on the activation of hematopoietic stem cells
-
Senolytic drugs and drugs that dampen mTOR reduce the burden of senescence
-
Periodic prolonged fasts mimic some of these effects associated with rapamycin
-
Secretions from senescent cells can affect the regenerative capabilities of stem cells
-
Cellular senescence may play a positive role in skin health
-
Different senescence phenotypes accumulate in different tissues
-
How telomeres protect DNA from damage
-
The surprisingly large effect of exercise on lifespan
-
The benefits of exercise in mitigating the side effects of chemotherapy.
-
The practicality of consumer assays for DNA damage
-
Vitamin B3 may improve tissue aging and mitochondrial function by reducing senescence
-
The effect of so-called fasting mimetic compounds on senescent cells
-
Elephants have extra copies of tumor suppressor gene TP53, reducing cancer incidence
-
Cellular senescence is a conserved mechanism we share with lower organisms
-
Programmed cell death as an alternative to senescence
Rhonda: Hello, everyone. Today I'm sitting here with Dr. Judith Campisi who is a professor of gerontology at the Buck Institute for Research On Aging. Judy has made significant contributions to the field of Aging, particularly for her research on cellular senescence, which I'm sure we're going to talk quite a bit about today. I've personally been following Judith's work for several years so I'm very excited to be able to have the opportunity to have discussion with her. So Judith...
Judith: Thank you. Thank you.
Rhonda: I'm ready to just jump in and talk about all things aging. You know, most people when they think about aging, they think about the passage of time, a chronological number, but there's a lot of molecular changes that occur with aging.
Judith: Yeah, well it's a biological process and it has its roots in evolution or it has its roots in actually, gestation. But it is not just the passage of time for sure.
Rhonda: So can you maybe talk about some of the molecular changes that do occur that can contribute to the aging process?
Judith: It's a good question. You know, right now, I think it's safe to say that most people who work on aging believe there are a few fundamental, molecular and cellular processes that drive aging in multiple tissues which is why all of the diseases of aging, even though they're from very different tissues all begin to rise exponentially after maybe 50, 60 years of age. But the truth is, we don't know exactly how many processes there are and we don't really know precisely in each tissue what the major driver is. So there are theories, the free radical theory of aging, loss of mitochondrial function, the accumulation of senescent cells which I'm sure we'll talk about more. But for now, I think there are two things we can say that these fundamental processes almost certainly are driving tissue health. As for what actually drives lifespan, we really don't know.
Rhonda: Yeah, I guess that's like an entirely different topic, I mean, to some degree.
Judith: I mean, they're related, nobody dies of good health. But the truth is that we don't really understand, for example, why a mouse lives three years and a human lives over a hundred years I mean, if you're lucky. And what it is that evolution had to do to take these two organisms, which are genetically pretty similar, but have a 30-fold difference in lifespan.
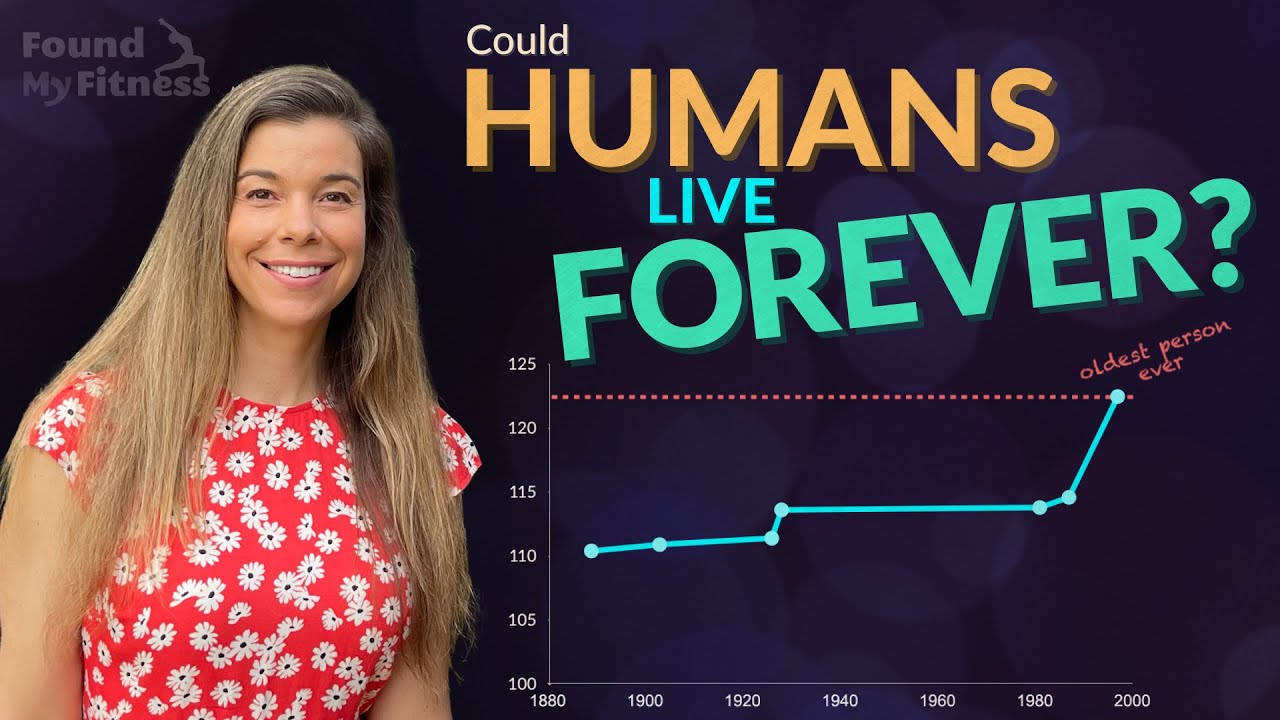
Rhonda: Right, yeah. Just kind of on the same topic, did you ever see that paper that was published? Maybe it was like 2015 I believe. It was out of Japan and the researchers had looked at a variety of different cohorts of aging individuals. So they looked at people that were considered elderly, so these were, like, 80 plus year olds, they looked at centenarians, they looked at the semi-super centenarians, and then the super centenarian. And they looked at a variety of different biomarkers. I believe it was something like 12 different biomarkers. They were looking at amino senescence in the immune system, but they were looking at telomere length, they looked at blood glucose levels, insulin sensitivity, inflammation markers and kidney function like all sorts of things in each going to age group.
Judith: Cohort.
Rhonda: Yeah, in each cohort. And what was found, sort of to my surprise but not too much to my surprise, is that the only going to biomarker that was indicative of successful longevity in every cohort was suppression of inflammation.
Judith: Yeah, I did read that. I did read that, yeah.
Rhonda: Whereas like telomere length affected if you could live to be, I don't know if it was 100 or the 80 group, one of the group, one of the you know, earlier groups but...so that was kind of interesting because it's sort of to me you know, this whole idea of inflammation which causes...
Judith: Inflammaging as Claudio Franceschi. No, this is the term that was coined by Claudio Franceschi in Italy, yeah. And it really refers to the fact that well, if a pathologist were to take a liver sample say from a 15 year old and a 50 year old, he or she could probably instantaneously tell you who was young and who was old. One would be just looking at the structure of the tissue but the other is he or she would look for what we call a low-level sterile chronic inflammation, meaning low-level infiltration of some immune cells but no evidence of the pathogen. And so this is a characteristic of almost all aging tissues is this low level inflammation.
Rhonda: Most people don't really, I mean, I think they hear the term inflammation, they don't quite know what it means.
Judith: Yeah. So they're two important...well, actually there are two major modes of inflammation, right. So one is acute inflammation. So you cut yourself, turns bright red, that takes 24 hours. And you'll die without that, I mean that's the acute response of your innate immune system that's going to try to protect you from infection but also start the healing process. That acute inflammatory response has to die down after a few days because it's destructive. The innate immune system isn't very intelligent. It's designed to kill non-specifically so these innate immune cells make hydrogen peroxide, nitric oxide, bleach.
Rhonda: Right, hypochlorite.
Judith: Exactly, the whole idea is just to kill non-specifically until your adaptive immune system can figure out what precisely needs to be killed. So that's your T cells and antibody response, but that takes a few days so you need both. Low-level chronic inflammation or sterile inflammation is similar to acute inflammation and that many of the players are the same but of course, it's at a much lower level. If it were that fulminant, you would probably die very quickly because your tissues would fall apart. But nonetheless, with aging, there's this constant increase in these innate immune cells that infiltrate your tissue. And after a while, the tissue does begin to degrade and lose its structure and lose its function.
Rhonda: Yeah, so it's not only you know, affecting the tissue, it's affecting going to DNA, it's affecting...
Judith: That's right because these are damaging agents and so it becomes like a feedback loop. You have going to cells that are being exposed to these mostly oxidants and yet, then DNA gets damaged and then the cell responds to the DNA damage and it makes things worse and you get into these feedback loops. So the question is what is the cause of that low level chronic inflammation? Of course, the short answer is we don't really know but we have some clues, we have some ideas. Some people have argued that with age, the barrier in your gut breaks down slightly. Not enough to cause bacteria to invade your tissues, but enough to enable bacterial products to leak into your bloodstream and then of course, your body is going to respond, "Oh, my goodness, is this an infection?" and so you get this slow buildup of immune responses. The other hypothesis, major hypothesis is that damage cells, which are what we now think are mostly senescent cells, are produced as part of their response to the damage. Cytokines which then attract the immune system and those are not mutually exclusive probably both.
Rhonda: Right, absolutely. Can you explain what, the people who are listening watching you know, what exactly a senescence cell is and how you know, it's obviously that you just mentioned it's producing more of these inflammatory cytokines and which to me sounds like it accelerates the aging process. But sort of just what is a senescence cell, why do they form?
Judith: Yes, very good question. So we now, we meaning the field now I think, would agree that senescence can best be understood as a stress response. It's the way cells have evolved a program to respond to certain types of stressors. And the result of that response, that stress response is two-fold. The first is that cells will lose the ability to divide, essentially irreversibly. So what happens is tissues begin to accumulate stress cells that can no longer divide. The curious thing is that they also tend not to die, or they don't very easily die so they accumulate with age. Very slowly and gradually, they're always at pretty low numbers but they go up with age.
The other part of that stress response is the cells turn on a program that causes them to secrete molecules that we classify as being bioactive. And the reason why that term is vague is because there are probably 50, 60, maybe 70 molecules that these cells begin to secrete and so they have many, many activities. Some of them are attract the immune system, so they're cytokines and chemokines that are immune attractants. Some of them are growth factors that will alert the neighboring cells, because maybe now if you have a need for proliferation the senescence cell itself cannot divide, but it may want to tell its neighbors, "Hey, start dividing." They secrete proteases that remodel the tissue that would also help with regeneration and repair. And they also, now we know very new data from our lab, they secrete bioactive lipids like prostaglandins and leukotrienes, which are very important for modulating inflammation fibrosis but also, again, tissue repair.
So the way we now think of the stress response is that it's a double-edged sword. There's tons of data that go back two decades or more showing that the arrest of cell proliferation is extremely important for preventing cancer. So that stressed cell is in danger of becoming a cancerous cell and the stress response intrinsic to the cell says, "No way, you will never divide again and therefore you cannot form a tumor." So there are mouse models now and even some people with mutations in the genes that regulate that growth arrest, and those people die in early death due to cancer, the mice die an early death due to cancer. So we know the growth arrest suppresses tumorigenesis.
The secreting factors we also now know can be beneficial for tissue repair and wound healing. So we've shown, for example, in the skin, senescent cells appear at the wound and they produce growth factors that help the wound heal. That's the good news. The bad news is, as these cells gradually accumulate with age and don't disappear, they begin to drive this process of chronic inflammation. And that chronic inflammation caused by senescent cells is also a double-edged sword. On the one hand, it attracts the immune cells that will eventually begin to cause tissue destruction and degeneration. On the other hand, the cytokines that attract the immune cells can also have an effect on neighboring cells and cause them to not function properly.
So for example, some of the cytokines that senescent cells produce cause what's called an epithelial to mesenchymal transition. So what is that? Most of the organs in your body are composed of epithelial cells and these are cells that absolutely must talk to each other. Mesenchymal cells are the support cells, like in the skin, the epithelial cells are the outer layer, the support cells are in the dermis. Those are the mesenchymal cells. They don't need to talk to each other, they need to signal. They're basically telling the epithelium what to do.
Now, when an epithelial cell becomes more mesenchyme-like, it stops talking to its neighbors and that means the tissue is going to start losing function. And so, senescent cells can change epithelial behavior so that the tissue doesn't function very well and it can attract the immune system which will cause then the destruction of the tissue do alienate immune cells. So that's the bad side. The good news is there are very few senescent cells in young people, and below age 50 or 60 you don't see very many of those cells in tissues but with after about the midpoint of our lifespan, they become detectable. And now, you can imagine that those cells as they build up, they start to drive the pathologies that we associate with aging.
Rhonda: Probably also start to cause more, yeah, cellular senescence. And so a couple of questions sort of popped up when you're talking about sort of the double-edged sword and how cellular senescence on the one hand, it's a function of a stress response that occurs to protect you from cancer and so the cell stops dividing. You know, and then, the senescent cells of course are then making all sorts of various different secreted proteins like you mentioned and that can have...sometimes, it can have good effects if you're talking about wound healing and things but also it can attract the immune system and cause you know, more...basically activate the immune system to make more of these hypochlorite hydrogen peroxide warfare things going on in the cell which can also damage the cell even more.
But the other thing is the growth factors that you mentioned that they're secreting and you know, let's say, as you are aging, you have accumulated more damage. And let's say now that that damaged cell is you know, supposed to undergo senescence because that's the stress response to protect you from cancer. But in the presence of excess growth factors, that doesn't actually always happen, right?
Judith: No, it can start the process of cancer.
Rhonda: So it's kind of ironic that various, the very thing that it's protecting you from it's also now at some point possibly now, making it more susceptible to at least as you get older.
Judith: Exactly, I mean, there's very good data now in mice from our lab and other labs that the presence of senescent cells can drive late-life cancer especially those cancers that are pre-malignant and poised to become cancerous but they don't have the mutations that are needed for a full-blown cancer. But under the stimulation, the growth stimulation of a senescence cell, these cells can now start proliferating, they pick up more mutations and then eventually, they become fully malignant.
Rhonda: Exactly. I think I read you did some experiments out of your lab where you guys injected these malignant cells into animals with and without the senescence cells.
Judith: That's exactly right and with senescent cells they converted to full-blown malignancy and you know, eventually kill the animal. So you know, you should be depressed, right? If you don't have this process, you die of cancer. If you do have this process you're still going to get cancer. I mean, we've struggled with this, we in the whole field have struggled. What do we do about this problem?
And the good news is, there are now mouse models. We don't know if this will work in humans, but in mice we can selectively cause senescent cells to die, so finally we can make them go away. And when we do that there are health benefits. I should point out, not necessarily an extension of lifespan but definitely an extension of health spans. So our lab has done this, our colleagues lab at the Mayo Clinic has done this. There are now a number of labs that are working on the strategy of causing senescent cells to die. And this was initially done with transgenic mice. Of course, we can't make transgenic people, but there's an army of people looking for drugs that will do what the transgenes can do. And some prototype drugs have been published. In mice, they're prototypes because they're not yet ready for people but you know, and there are companies that are working on these things.
So the good news is I think the strategy of tuning down the bad effects of senescence is on the way. That's on the horizon for sure. We will still have to be careful how we apply those drugs, right? If you're going in for surgery you don't want to take drugs that will kill off senescent cells because you need to heal the wound from the surgery. So there's going to have to be some intelligent discussions about when it would be appropriate to take these drugs. The good news about the drugs though is that, from the mouse models, it's not as though senescent cells you know, blossom at some point after some age. They just gradually accumulate. So what that means is you don't have to take a drug that kills senescent cells every day. You may have in mice you can apply them every few months and in people, maybe every few years. So this could be interesting strategy to improve health by incrementally knocking down senescent cells every so often.
Rhonda: Yeah, so you said that with these studies that cleared away the senescence cells, the health span was improved, meaning I guess the tissues, the organs aged better and things like that. But I thought I remember a study, maybe it was the Mayo Clinic study where there was a mouse model. Perhaps, it was an accelerated aging mouse model where they did have a lifespan extension of like 20%.
Judith: No. So that was an increase in median lifespan...
Rhonda: Median lifespan, okay.
Judith: ...but not an increase in maximum lifespan. And I should have distinguished between median lifespan and maximum lifespan. So the increase in maximum lifespan was not significant. The increase in median lifespan was significant and so that's what we call health span. The animals still died but they were healthier in many respects. You know, it's interesting so what did they die of? It's not as easy as it might seem to determine what kills the mouse. Actually, even in people sometimes going to pathologists put "Heart stopped." We don't know.
Rhonda: Yeah, we don't know, okay so there was an increase in the median lifespan which is still very important because now you're having more of these animals which are living longer but they're....
Judith: They are living healthy.
Rhonda: ...healthier, so right.
Judith: And of course for humans this is amazing if you...I mean, what people dread about old age is those last years of life when going to you go through a nursing home and people are, they're barely mobile and you know they can't hear, and they can't see, and they're depressed for good reason. But you know, I think the image now of being able to be vibrant and then who knows what will do you in the end, but you know, we will die but I think what we mostly fear is those last few years of life where the quality of life is very poor.
Rhonda: Where you're degenerative, and you know, a lot of these diseases that are degenerative diseases aren't necessarily like going to kill you right away or going to as you said you could have you know maybe macular degeneration or sarcopenia. It's something where it's just a little slow and miserable and you can't function as well.
Judith: And then brain going to degeneration of the brains. I mean, dying of cancer is no fun but I think many people fear even more, this loss of cognition. It's like you lose yourself and then the incredible burden on the family. And so the idea is to try to compress that period of morbidity so that the last years of life going to you die maybe have a heart attack on the tennis court and you're winning. There's a lovely quotation from Thurgood Marshall. He was our first black Supreme Court justice, this was in the 1960s and someone had the nerve to ask him how long he plans to live, a lifetime appointment right, you know. And he said, "I plan to die at 110 from a bullet wound from a jealous husband."
Rhonda: That's pretty funny. Talking about the brain, you just mentioned the brain. I've always been curious about what tissues...if certain tissues accumulate more senescent cells than other tissues, including in the brain.
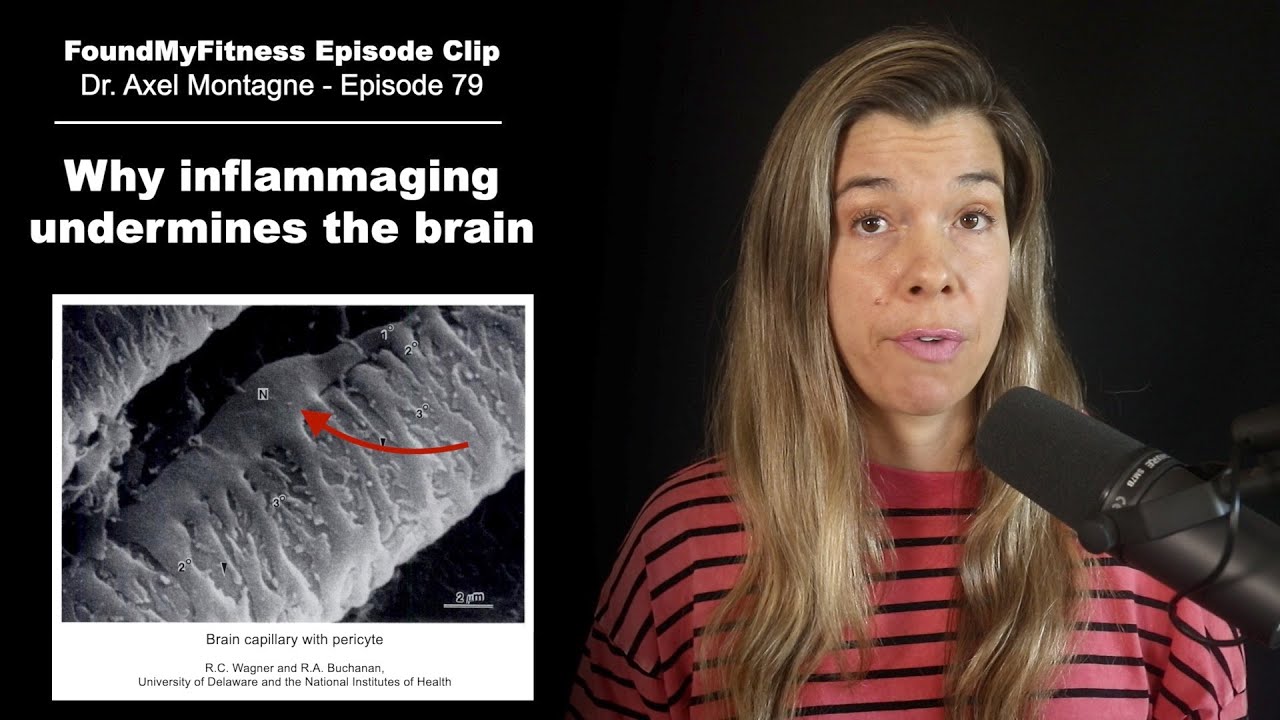
Judith: Yeah. So we and others have looked at senescence in the brain. Based on the markers we have, so I should also preface this by saying, we don't have perfect markers for senescence cells. There are many markers, and so we tend to have some confidence that we say a cell is senescent if we look at multiple markers and we say, "Well, it's a good chance, this is a senescence cell." So the best markers that we have have been used in the brain by a number of laboratories and it seems that the cells that are more likely to become senescent in the brain are astrocytes.
Rhonda: Splitting cells.
Judith: Exactly and so that's interesting from two points of view. The first is there are probably more astrocytes in your brain than neurons. Yeah, people don't realize that but going to there are lots of astrocytes. The second is it's the astrocytes that give rise to brain cancer. So again, consistent with the idea that the stress response protects us from cancer at least for a while. We've studied human astrocytes and I can tell you they amount to classic senescence response including producing all those pro-inflammatory cytokines, and growth factors, and proteases. And we even have new evidence that astrocytes as you know, help protect the neurons from certain types of toxicity like neurotransmitter toxicity, and we can show that when astrocytes become senescent they become less effective in that protective response. Yeah, so again you know being able to get rid of those senescent astrocytes could be beneficial for preserving brain function. Now, once neurons die, that may be too late so we have to distinguish between the ability to prevent degeneration in the brain versus reversing it. I think reversal is going to be much harder.
Rhonda: It's always much harder for reversal.
Judith: Yeah, yeah.
Rhonda: Is the senescent cells that occur in the astrocytes, do you think that also going to because as we age brain inflammation also increases. Do you think it's a contributing factor?
Judith: Yeah, we definitely think that's a contributing factor. It may not be the only factor, but definitely.
Rhonda: Well, there's now going to lots of evidence showing that you know, a lot of cytokines in the periphery can cross the blood-brain barrier with a variety of mechanisms, including this newly discovered lymphatic system that's connected to the brain, the meninges or something.
Judith: Exactly, that's right. So that's interesting. So you don't necessarily even have to have astrocytes senescence to help contribute to neurodegeneration. It could be in your liver or your skin.
Rhonda: Exactly, exactly. So sort of like just sort of all connected. Maybe we can talk a little bit about the mechanisms that lead to cellular senescence. I mean, we mentioned inflammation, sort of, you mentioned it's a stress response, it's just the stress but you know specifically...
Judith: What are those stresses?
Rhonda: What does are the...like I know the main mechanism I always think of when I think of cellular senescence.
Judith: DNA damage?
Rhonda: DNA damage.
Judith: Yeah, for sure, anything that causes severe or persistent damage to the genome will drive cells into senescence. It makes sense because that puts you at risk for mutations, mutation puts your risk for cancer, so the cells want to shut that damaged cell down. But there are other stresses now that we know. We recently showed, for example, that having bad mitochondria in the absence of DNA damage...so this is just mitochondrial dysfunction if you will. And we could show that you could cause mitochondrial dysfunction by any number of means. Five or six different ways of causing the mitochondria to fail to produce the energy that they need and to produce more free radicals, even in the absence of those free radicals getting to the nucleus cause the cells to senesce, so they will senesce in response to bad mitochondria.
What's interesting is the cells senesce, they stop dividing. They do start secreting molecules but it's a different complement of secreted molecules. So we can now pretty much determine whether a cell has become senescent due to DNA damage versus bad mitochondria based on their secretory profile. There's overlap, don't get me wrong there's overlap but there's also distinct features that the mitochondria are responsible for, bad mitochondria are responsible for versus damaged DNA.
Rhonda: What would you say the main distinctive features?
Judith: So one of the main distinguishing features is with DNA damage, there's a pathway that increases cytokines like IL-6, IL-8, these are very prominent pro-inflammatory cytokines. That doesn't happen with bad mitochondria so that loop is pretty much not activated. But then other molecules are activated that can also be pro-inflammatory but through a different pathway.
Rhonda: Wow, so this, kind of, is very extremely interesting. I wonder why that is and I wonder what, like, if there's different going to functions of these senescent cells. But the secretion of some of these pro-inflammatory cytokines and the attraction of immune cells to that going to to that area, one would think would then cause that senescent cell to be cleared away.
Judith: Yes, yes, and it probably does happen. But it probably doesn't happen efficiently enough, or with age maybe we make senescent cells at a higher rate. So several labs, not our lab but several other labs have shown that senescent cells express molecules on their surface that can target them for being killed by the immune system, and the immune system can do that and it does do that. Nonetheless, we still see this increase with age.
Rhonda: But the immune system declines with age, right, function of the immune system does. Do you think that may contribute?
Judith: Well, that's a big unanswered question. So what happens with so-called immune senescent is primarily the adaptive immune system.
Rhonda: That's actually the good part of it.
Judith: Exactly, exactly and this is why you become more susceptible to certain types of infections with age. So the adaptive immune system tends to decline with age. The innate immune system if anything increases in activity with age and it's the innate immune system that clearly targets senescent cells for clearance. So for example senescent cells express on their surface ligands for natural killer cells and natural killer cells will then attack those senescent cells and kill them. Nonetheless, senescent cells still accumulate with age.
So we're considering several possibilities. So one is maybe you're just making them too fast, the immune system can't keep up. The other is that although the innate immune system doesn't decline with age per se, it does change and it could change in a way that it becomes less efficient at clearing senescent cells. So that's a big open question, we don't know the answer to that. And the third possibility is that some senescent cells may develop mechanisms to protect themselves from immune clearance and we're sort of still studying that now. And we think maybe all possibilities are still open.
Rhonda: Geez, I have so many things that I want to talk about it which order because I'm going to forget. So talking about these changes in the adaptive versus the innate immune system sort of reminded me of Dr. Valter Longo's research, who I interviewed a few months ago. And he was talking about how this prolonged fasting in mice, which is about 48 hours or translates to like four days in humans which is quite a long fast, but was able to just very robustly clear away damaged cells, presumably senescent cells well. Also caused cellular death, but followed by a massive and robust increase in stem cell proliferation sort of replenishing the population. But what was so interesting was that, it seemed to at least in aging mice, if you did this in aged mice, it normalized the difference between the innate and the adaptive. So like you were mentioning, the adaptive immune system declines with age, but this fasting sort of like replenished somehow I guess the adaptive maybe some of the stem cells.
Judith: In the bone marrow, yeah, yeah.
Rhonda: Yeah, so you maybe have more of a 50-50 ratio like you do when you're younger. So what would be interesting is if you like somehow did that experiment where you caused going to disinvesting and going to sort of regenerated that adaptive immune part, I guess of the immune system. And then like gave some chemical to cause senescence and see if there's any change in...
Judith: Well going to a lot of Valter's work also has to do with the side-effects of chemotherapy, right. So he was able to show that with this intermittent short-term fasting, even in humans, he could not only improve the efficacy of chemotherapy but prevents some of the side effects. So we've shown very recently using mice, a transgenic mouse model, that some of the so-called genotoxic chemotherapies, chemo therapies that damage DNA, definitely causes senescence. And if we eliminate those senescent cells in our transgenic mouse model we can eliminate several of the side effects, several of the bad side effects of chemotherapy.
So one possibility is that what the fasting does is it might eliminate senescent cells. More likely what it might do we think is dampen mTOR activity. So just to remind you, mTOR is the kinase which is highly conserved from yeast all the way to humans and it's a nutrient and growth factor sensing kinase, so high nutrients, high TOR activity. And what has been shown in yeast, and worms, and flies, and mice is that if you dampen...you can't get rid of TOR activity, you need it for life. But if you dampen TOR activity either genetically or with the drug rapamycin, which is known to target one arm of the TOR pathway, you can extend lifespan. And what we showed recently is that what rapamycin does or dampening TOR activity does is it also suppresses primarily the inflammatory arm of secretory phenotype of senescent cells. So it could be that fasting, and rapamycin, and the secretory phenotype of senescent cells all come together around the TOR pathway, and that it's really TOR activity that's driving both the aging phenotypes, the side-effects of chemotherapy, and may partly explain the benefits of the short-term fasting that Valter is a proponent of.
Rhonda: Interesting. So if it's dampening the secretion of these cytokines from the senescent cells but it's not actually getting rid of the senescent cells.
Judith: It's not so far as well, we've shown that either genetically or pharmacologically with drugs like rapamycin suppresses the secretion of senescent cells but it doesn't kill them. So unlike some of these other drugs at least your so-called senolytic drugs that actually kills senescent cells, the mTOR drugs, the mTOR dampening drugs suppress the ability of the senescent cells to secrete. And the effects last longer than the application of the drug in the sense that we know that part of that secretory pro-inflammatory secretory phenotype is due to a feedback loop. And what dampening mTOR does is it breaks the loop and the loop takes time to reestablish. So even after you withdraw the drug, you still get suppression until eventually the loop reestablishes and then the cell starts secreting again. So you might think that if you were to fast for four days going to every few weeks, you might have more benefit, certainly more benefit than taking a drug like rapamycin which has side effects.
Rhonda: Right because you're not only going to when you're fasting, you're also clearing away the damaged cells in theory. I mean, we don't know how much of that's occurring in humans yet, we do know, in mice that happens.
Judith: Yeah in mice, that happens.
Rhonda: And then the other thing, this kind of goes back to the mitochondria induced senescence that we talked about a minute ago is that we know that fasting increases NAD levels and so the NAD plus, NADH ratio. And that sort of is very interesting because this mitochondrial induced damage, I think has something to do with declined immunities.
Judith: It does actually. So that's what we've shown is that this mitochondrial dysfunction induced senescence we call it mitochondrial dysfunction associated senescence or MiDAS. So we call it the MiDAS phenotype. It really has to do with this altered NAD, NADH ratio, and that's one of the drivers. Interestingly, so when you change that ratio, you activate a kinase called AMP kinase. AMP kinase is a major regulator of p53, p53 is a major regulator of both senescence and apoptosis. So that could be the link of why in Valter's paradigm, you get reduced inflammation that could be due to suppressing the secretory phenotype but also increased apoptosis because you now have activated p53.
Rhonda: Right, exactly. Yeah, so just got sort of lost and the question I was going to ask you but the...
Judith: One of them is going to...yeah, I don't know if you can ask this question but it's worth discussing is the stem cell, the regenerative process that Valter has seen. So we also know that the secretions of senescent cells can have very profound on stem cell proliferation and function. So it could also be that by dampening the secretory phenotype of senescent cells, you now release those stem cells from the suppression that was due to those secretory phenotypes and therefore allow them now to do what they do best, which is to proliferate and regenerate a tissue. So all of things really probably tie in to each other, I mean they're all interrelated.
Rhonda: So the growth factors that are actually secreted by the senescent cells do help with stem cell growth? Because of senescent cells like if this happens in a stem cell, you're depleting the stem cell pool and it's contributing to the stem cell aging.
Judith: Both are probably true, both are probably true. So we have shown in the skin, for example, that with age, senescent cells do accumulate. But if you clear those cells you don't get much benefit, and that's because by old age you've depleted the stem cells. So once you've depleted that stem cell pool, you can't go backwards or at least you can't very easily go backwards. But two labs have now shown that senescent cells can also produce growth factors or factors that help neighboring cells reprogram to stimulate regeneration and they do it again by their secretory phenotype. So it's again this double-edged sword. Some of the secretions of senescent cells dampen stem cell activity and others promote stem cell activity.
Rhonda: So the mitochondrial induced senescent cells, what's their function? I know the DNA damaged induced ones are you know, obviously are protecting from cancer. What's the purpose?
Judith: What is the evolutionary purpose?
Rhonda: Yeah.
Judith: Well if you have a cell with bad mitochondria, you probably want to clear that cell, prevent that cell from propagating, because then you're going to have clones of cells with bad mitochondria. And we know that that causes all sorts of degenerative diseases, neuro degeneration as well as muscle degeneration. And so it probably is also protected but not so much against cancer but against accumulating degeneration within a tissue. We know for example that people who are born with mitochondrial DNA defects, eventually the bad mitochondria expand and so that's not good for an organism. So there probably is a protective mechanism to prevent the propagation of cells with bad mitochondria.
Rhonda: Okay, well that makes more sense. I guess going to at a certain point, your mitochondria, if you don't have you know, a really bad defect in mitochondrial DNA, your mitochondria will repair themselves through fission/fusion. I mean yes, right, isn't fusion also part of how this exchange all their mitochondrial content and the damaged one sort of fixes itself to some degree? Although, I guess that gets diluted.
Judith: The bad ones also get eaten up by the lysosomes.
Rhonda: Right, yeah so I guess there's multiple mechanisms. Do the mitochondrial induced senescent cells, they produce the growth factors that affect stem cells?
Judith: They produced some growth factors yes, they produce for example, amphiregulin, which is a EGF-like growth factor. So they do, they do. So where we're still struggling with trying to understand you know, what aging phenotypes are caused by genomic damage, what aging phenotypes are caused by mitochondrial...
Rhonda: Muscle atrophies. Do you find more muscle tissue, mitochondrial induced?
Judith: Well, we find more senescent cells and we find them in the heart but we don't know how they got there. It's a big unanswered question as to when you see a senescent cell in vivo, which we do in human tissue and mouse tissue with age, they accumulate but what caused them to become senescent? Is it mitochondrial damage, is it genomic damage, is it metabolic imbalances? We really don't know yet, it's still one of the big unanswered questions.
Rhonda: Yeah, that sort of leads me into the preventive sort of questions I had and that is you know, we do know that there are lifestyle factors that affect mitochondrial health, that affect DNA damage, that affect telomere length going to and these things are obvious to a lot of people. I mean, a lot of work out of Elizabeth Blackburn's lab with Elissa Epel, they've done some really great work and a lot of its associative studies where they're looking at telomere length and various lifestyle factors but they've shown for example going to that exercise is very important and people that are that are sedentary have shorter telomeres than people that are physically active. People that are stressed have shorter telomeres. Actually, people that have low vitamin D have shorter telomeres, omega-3, sugar accelerates the aging process at the level of telomere. So all these factors and telomere length also is a major regulator of cellular senescence.
Judith: Oh yeah, I think the best way to think about telomere length is that when the telomeres become too short...so telomeres form a structure that caps the ends of the chromosomes and makes sure that the cell doesn't mistake that chromosome end for broken DNA. Because the cell will try to fix that broken DNA by fusing it to another broken end, and you don't want that to happen with your telomeres. So when the telomeres become too short, that cap structure then is disrupted, and now the cell thinks it has a broken DNA and it will try to fix it if it can, but if it can't it will cause senescence. So short telomeres are in a way a subset of DNA damage which again is a major driver of senescence. So I mean, telomeres tie in exactly to the whole thing.
Rhonda: Don't telomere sort of take the hit for DNA damage as well?
Judith: Well, they have a fairly high proportion of the nucleotide guanosine, right? And that base is pretty susceptible to oxidative damage, so it becomes like a sensor for damage. It's not the only thing that will cause DNA damage that is oxidized guanine but telomeres are good sensors of whether there's oxidative damage.
Rhonda: Do you agree that things that at least have been associated with DNA damage and telomere length in humans and associative studies may likely be the same things that help prevent the accumulation of cellular senescence?
Judith: Yes, yeah, to some degree exactly. I mean, I don't want to oversell the idea that senescence can explain all of aging. It's like telomeres can or DNA damage can't. But we think it's an important process and it's tied in to other things that may intersect with the senescence pathway and including things like telomere length and genomic damage but also things like exercise. So there's a recent study that I thought was really interesting. This was a study that looked at lifespan longevity in obese people. So both groups were obese, but they compared obese people who were sedentary with obese people who were moderate exercisers. And there was something like an 8-year difference in life span.
Rhonda: You're kidding.
Judith: No, even with the obesity, moderate exercise in general protected the longevity of obese people. And so, imagine what it does for people who are not obsessed. I think exercise is probably the single most important intervention that cuts across multiple diseases. So sarcopenia which is going to muscle loss with age major cause of going to you see people sitting in wheelchairs like this, it's horrible. The only intervention, effective intervention is exercise. And we know that exercise can have some effects on senescent cells in vivo but we don't know how it works, we don't know precisely what it does.
Rhonda: What about the stress response pathways it activates? I mean, exercise is a type of hormetic stress so it's a little bit stressful and activating all these.
Judith: Yeah so that's one idea is that it's hormetic stress meaning it's low level stress that then primes everybody else, all your other stress responses to be hyper-vigilant. And so then when a bad telomere comes along, or an insult from radiation or high sugar because you just couldn't resist that last brownie, you're better able to deal with that stress. It's one hypothesis. I think there's still you know, an ocean of ignorance around how exercise seems to be so beneficial for so many indications of aging. Again, aging not necessarily maximum lifespan, healthspan.
Rhonda: Yeah, it does seem to affect many different diseases of age as well in addition to sarcopenia, cardiovascular health. I mean going to cardiorespiratory fitness is also very tightly correlated with longevity.
Judith: And there are some groups now that are studying the effects of exercise on side-effects of chemotherapy and showing benefits of again, mitigating some of those side effects simply by an exercise regimen. And we're not talking running marathons, we're talking sort of moderate but persistent exercise.
Rhonda: It's fascinating. I've read a couple of the studies, at least the animal studies where they can sort of force them to run a little bit more on this running wheel and it is like there was a very robust response in terms of at least in combination, I think with the standard of care treatment where I want to say something like 50%. It was something very like, really you know, like that's very...you know, I've always thought about it as sort of when you're exercising you're forcing your mitochondria to work harder and you're producing more reactive oxygen species and cancer cells don't like that. I mean going to so who knows?
Judith: Yeah, I think yeah, mechanism so far unknown but lots of possibilities.
Rhonda: Right what about the hope for a clinical assay for measuring things like cellular senescence or at the very least, DNA damage in people?
Judith: Yeah so that can be done. I mean, I know of at least a couple of companies that are doing this. Usually, they use peripheral blood and there are very good antibodies that will detect persistent DNA damage. So you can you can stain these blood cells and get a sense of what your DNA damage load is. There are good markers for senescent cells now. We always recommend that you use two or three but we can probably assess the load of senescent cells. The difficulty lies in tissue specificity. So peripheral blood is easy going to buccal swabs are easy because they're accessible. But you know, you really probably want to know how many senescent astrocytes you have in your brain, and you know, brain biopsies are not going to be approved very soon. So that's the difficulty is we can get a general idea from those easy to access tissues but there are tissues we might want to know about that are not going to be easy to biopsy.
Rhonda: Is there a correlation between let's say, if you were to look at cellular senescence in white blood cells or leukocytes between that and the heart or the brain?
Judith: Yes, yes there is, and of course the work that really exemplifies at the most is the work on telomere length where you take you know, peripheral blood that's mostly what's being used now to assess you know, telomere length. But you look at the data and of course there's enormous scatter in the data, there's always a young person you know, who's down with the length that's equivalent to a 90 year old and 90 year old who's up there with the equivalent of a 16 year old. And part of that I'm guessing is due to the fact you're measuring one tissue and you don't know what the history of that. You know, when was the last time you had a cold? That's going to affect you know, how many t-cells you have with bad telomeres or not. And so human data tends to be messy in that sense.
Rhonda: It's very messy. I've actually seen this quite a bit because I've done a lot of work with Dr. Bruce Ames and I've measured DNA damage in people and lean people, obese people, after a certain you know, giving them an insert intervention and I do this by measuring phosphorylated H2AX. But I've seen even looking at different age groups like you know, sometimes like the 20 year old will like look like a 70 year old and you're like, what happened going to here? And sometimes the obese people look really great but most of the time obese have higher levels than lean. But there's certainly there's a lot of variation. There's a lot of variation.
Judith: Yeah, and I think that variation is two-fold. The first is you know, we're people. We are not genetically identical the way our mice are. So there's going to be individual-to-individual variation because we're not genetically identical. But the other interesting aspect is that we've taken in our mice...we have transgenic mice in which senescent cells activate a protein, a luciferase that we can then measure by luminescence in the whole animal. So we can follow the appearance of senescent cells in living animals by looking at this luminescent signal.
So we start with say 12-month-old mice, so that's a 35 or so year old person, very low signal. And then as these animals age, the luciferase signal goes up, and up, and up. Then you look at the error bars. Genetically identical animals sometimes in the same cage and the error bars get larger, and larger, and larger. So that says there is stochastic variation that's not due to genetic differences that causes identical animal to have some with a high burden of senescent cells, some with a low burden of senescent cells. And this is true for virtually every aging marker that has been looked at. The error bars get larger, and larger, and larger. So, we call this stochastic variation. We don't know whether it's malleable, meaning we don't know whether you could make things less variable or more variable. We don't know whether it correlates with health of the animals, but it is a very common feature of aging is that things that go wrong do it almost randomly.
Rhonda: And there's no idea of what's causing it?
Judith: Well, I mean, you know that if you take a bunch of cells, identical cells, genetically identical cells and you apply some toxin and then you look at damage, you get a Gaussian curve, meaning there are some cells that don't respond very well. Most of the cells respond a certain way and then some cells that are super responders. And so it probably is just the messy nature of biology. We have all these pathways that are intertwined, and by chance one configuration makes the cells super responsive and another configuration makes a cell less responsive and if that's true of cells, and it's definitely going to be true of something as complicated as a mouse, much less a person.
Rhonda: Right. so I have just a couple more questions, couple more wacky but just a circle back walk, since I have you here, I know we've been talking for a while. But back to the NAD levels that reminded me of this whole new field as sort of semi new, I guess on you know, different precursors of NAD that you can get like nicotinamide riboside, nicotinamide mononucleotide. I know nicotinamide riboside at least in humans has been shown to increase NAD levels at least at very high levels. What do you think? Do you think that any of those, and I know that there's been some animal studies showing it increased health span so that the tissues were aging and some organs were aging better. Do you think that any of those effects of the increased energy had to do with lower cellular senescence?
Judith: That's a good question. As I mentioned, we know that this mitochondrially driven senescence, it's definitely driven by this alteration in the NAD-NADH ratio so it's possible. We haven't really studied those precursors directly on senescent cells, but yeah.
Rhonda: It's interesting.
Judith: Sounds like a grant I should write.
Rhonda: Yeah, there you go. And then one more question about before I get to the other wacky question was what do you think about some of these, like people call them, "Fasting mimetics" I don't like that. I think there's too many things going on with fasting, but you know, that have been shown to clear away damaged cells like spermidine or the hydroxy citrate, or resveratrol. Do you think that's something? I mean people are taking these to like clear away senescent cells.
Judith: Yeah, we've explored some of those. Some of them have no effect on senescent cells which doesn't mean that they might not have health benefits but it just doesn't act through senescence. Resveratrol for example, we don't see any effects on say pro-inflammatory cytokine secretion or anything that we think might be important, but that doesn't mean that it's not doing other things. I much prefer red wine. Cells don't like it.
Rhonda: Cells don't like it. The other wacky question that just came to my mind was, so I'm thinking about cellular senescence, not the mitochondria cellular senescence but you know, the DNA damage induced cellular senescence, as a protective effect against cancer and that's why it's evolved. I mean, we have that because we don't want to die of cancer young. What about animals like elephants? They have a relatively long...
Judith: I don't think it is that they don't get cancer, I think what's amazing is that they're so big, right, and they have so many cells. And so you would think that there should be ways, there should be super ways they have of protecting against cancer because it's a hell of a lot of cell division to go from a single elephant egg and sperm, you know a zygote going to to an elephant which is so big. And there have been some studies on looking at for example, tumor suppressor mechanisms in some of these animals. P53 right, they have extra copies of p53.
Rhonda: Do they have any cellular senescence? I was just wondering if anyone's looked at it?
Judith: I don't know that anyone has looked but I would be surprised if they don't. I mean, we've seen cellular senescence in, or we, meaning the field, has looked in a number of vertebrate species and it seems to be common amongst all vertebrate species.
Rhonda: Does it occur in lower organisms? They only live in weeks?
Judith: Well it does. Based on the markers we have some people have looked in C. elegans and they don't seem to find it there but then, C. elegans is unusual in that the only dividing cells and the worm is the germ line. But in Drosophila going to there is a small fraction of cells that undergo division in the gut and there is some hints that there may be senescence that occurs in the gut of the fly and that kind of makes sense because one thing that happens with age in the fly is they get this gut hyperplasia, it's almost like colon cancer in the fly. But they don't have the vast number of dividing cells that say, we have, our a mouse has. So it's possible that it might be found in Drosophila.
Rhonda: Okay, and what about that...just now one triggered a question. Does cellular senescence occur in more rapidly proliferating cells? Is that like the gut in a human?
Judith: It can. I mean, the gut is different I think because remember, those cells are programmed to die.
Rhonda: Right, kind of like the immune system I guess?
Judith: Or some of, yeah, some immune cells. I mean, but if you think about say something like the skin so again, those cells are programmed to die but then there are these basal epithelial cells. It takes a long time. So unlike the gut where there's very rapid sloughing off of those cells takes longer in the skin. And of course people do get skin cancer and they always come from the basal keratinocytes. This is certain types of skin cancer, right, not all. I mean, not melanoma for example which comes from melanocytes. But so those cells can transform even though they're programmed to die and we do see senescence in the basal layer of keratinocytes in human skin. So maybe.
Rhonda: Does that contribute to collagen breakdown and other stuff?
Judith: Well that's the idea is that it could because they're making a lot of proteases that will destroy collagen. Yeah, but I mean going to nobody really knows, yeah.
Rhonda: And so, with all your research that you found you know, throughout the years on cellular senescence and just aging in general, do you have any practices that you have sort of gleamed from your own research that you incorporate into your own lifestyle?
Judith: Yeah, so you know, moderate exercise, although I always seem to be busier than I like to be. You know, a good diet. I don't smoke, eat your veggies but then there's this genetic component which says you should choose your grandparents very wisely.
Rhonda: And that's sort of difficult to do. So Judith this has been a very illuminating conversation. I really enjoyed it.
Judith: Thank you for your interest.
Rhonda: Very interesting and I look forward to I'm going to continue reading about your research, I can't wait to learn about all the new things that you discover. But for people that want to learn more about your research, do you have a website or just the Buck Institute?
Judith: Yes, they can go to the Buck Institute website and you know, there's websites for the individual faculty members.
Rhonda: Right, I know if you just google Judith Campisi, The Buck Institute's like the top hit so they can learn more about your research there.
Judith: Yes.
Rhonda: Okay, well thank you, Judith.
Judith: Thank you, thank you.
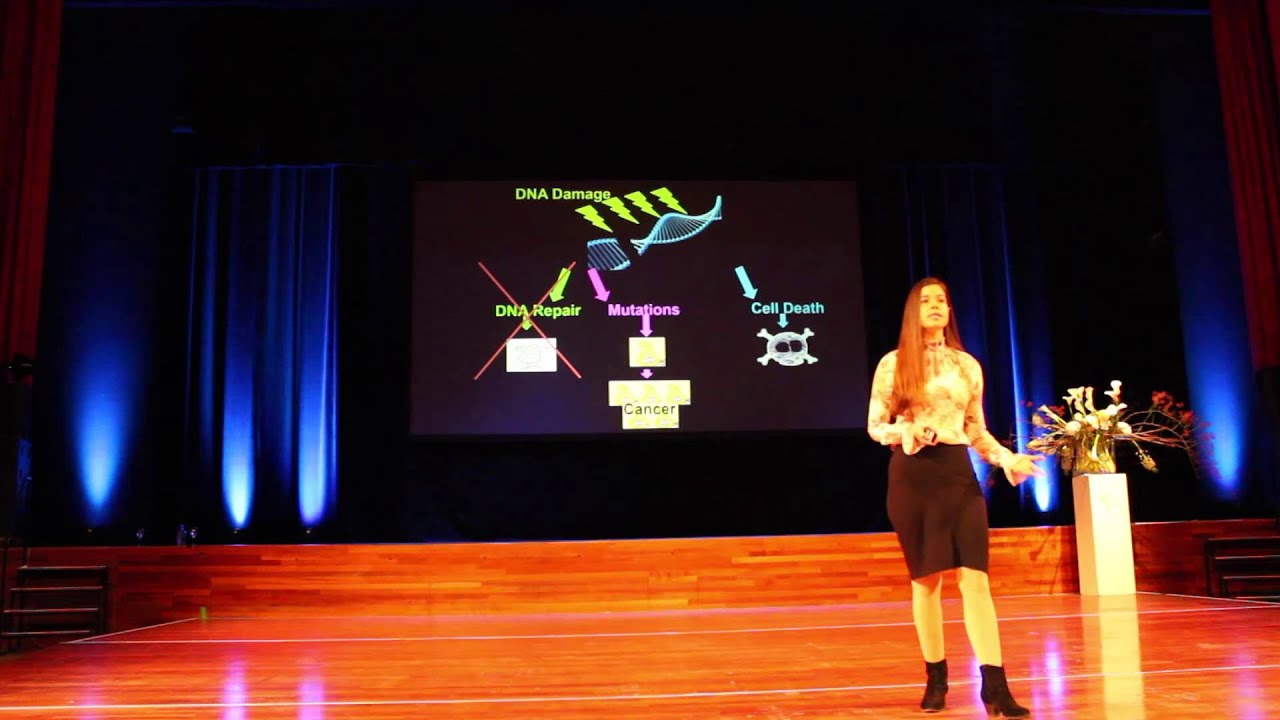
As cells become nonfunctional, they produce inflammatory cytokines and compromise tissue health.
A protein that serves as an autocrine growth factor and mitogen for astrocytes, Schwann cells, and fibroblasts. Amphiregulin is related to epidermal growth factor (EGF) and transforming growth factor alpha (TGF-alpha). It interacts with the epidermal growth factor receptor (EGFR) to promote the growth of normal epithelial cells.
An enzyme that plays multiple roles in cellular energy homeostasis. AMP kinase activation stimulates hepatic fatty acid oxidation, ketogenesis, skeletal muscle fatty acid oxidation, and glucose uptake; inhibits cholesterol synthesis, lipogenesis, triglyceride synthesis, adipocyte lipolysis, and lipogenesis; and modulates insulin secretion by pancreatic beta-cells.
Programmed cell death. Apoptosis is a type of cellular self-destruct mechanism that rids the body of damaged or aged cells. Unlike necrosis, a process in which cells that die as a result of acute injury swell and burst, spilling their contents over their neighbors and causing a potentially damaging inflammatory response, a cell that undergoes apoptosis dies in a neat and orderly fashion – shrinking and condensing, without damaging its neighbors. The process of apoptosis is often blocked or impaired in cancer cells. (May be pronounced “AY-pop-TOE-sis” OR “AP-oh-TOE-sis”.)
A test used in laboratory medicine, pharmacology, environmental biology, and molecular biology to determine the content or quality of specific components.
Star-shaped cells found in the brain and spinal cord. Astrocytes facilitate neurotransmission, provide nutrients to neurons, maintain neuronal ion balance, and support the blood-brain barrier. Astrocytes also play a role in the repair and scarring process of the brain and spinal cord following traumatic injuries.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A highly selective semi-permeable barrier in the brain made up of endothelial cells connected by tight junctions. The blood-brain barrier separates the circulating blood from the brain's extracellular fluid in the central nervous system. Whereas water, lipid-soluble molecules, and some gases can pass through the blood-brain barrier via passive diffusion, molecules such as glucose and amino acids that are crucial to neural function enter via selective transport. The barrier prevents the entry of lipophilic substances that may be neurotoxic via an active transport mechanism.
A transparent nematode species (a type of roundworm), about 1mm in length. The first multicellular organism to have its whole genome sequenced. Because they have a short lifespan (about 14-15 days), they are a good model organism for aging research. Strains are inexpensive to breed and can be frozen. When subsequently thawed, they remain viable, allowing long-term storage.
A highly regulated process in which a single cell replicates its genetic material and then splits to form two identical cells, sometimes referred to as “daughter cells.” Cell division, also known as mitosis, occurs in five distinct phases: interphase, prophase, metaphase, anaphase, and telophase, known collectively as the “cell cycle.”
A person who is 100 or more years old.
A tightly coiled molecule of DNA found in the nucleus of a cell. Chromosomes contain the genes and other genetic material for an organism. Humans have 46 chromosomes arranged in 23 pairs. Each chromosome is comprised of long stretches of DNA wrapped around proteins called histones, which provide structural support. At the end of each chromosome is a repetitive nucleotide sequence called a telomere. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division.
A broad category of small proteins (~5-20 kDa) that are important in cell signaling. Cytokines are short-lived proteins that are released by cells to regulate the function of other cells. Sources of cytokines include macrophages, B lymphocytes, mast cells, endothelial cells, fibroblasts, and various stromal cells. Types of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factor.
A major contributing factor to aging, cellular senescence, and the development of cancer. Byproducts of both mitochondrial energy production and immune activity are major sources of DNA damage. Additionally, environmental stressors can increase this base level of damage. DNA damage can be mitigated by cellular repair processes; however, the effectiveness of these processes may be influenced by the availability of dietary minerals, such as magnesium, and other dietary components, which are needed for proper function of repair enzymes.
A genus of flies, often called "fruit flies," that has been heavily used in research in genetics and is a common model organism in developmental biology. Fruit flies are popular experimental animals because they are easily cultured en masse out of the wild, have a short generation time, and mutants are readily obtainable.
The transdifferentiation of epithelial cells into motile mesenchymal cells, a process known as epithelial–mesenchymal transition (EMT), is integral in development, wound healing and stem cell behaviour, and contributes pathologically to fibrosis and cancer progression.
Cells accumulate free radical damage over time. These radicals can be generated from sources such as oxidative respiration in mitochondria or as collateral damage as a consequence of immune responses.
The phosphorylated version of histone 2A that forms when double-strand breaks in DNA occur. Formation of gamma-H2AX acts as a signal for DNA repair enzymes to be recruited to the site of damage in order to repair it. Gamma-H2AX is a biomarker for DNA damage.
The scientific study of old age, the process of aging, and the particular problems of old people.
A naturally occurring substance capable of stimulating cellular growth, proliferation, healing, and differentiation. Growth factors typically act as signaling molecules between cells. Examples include cytokines and hormones that bind to specific receptors on the surface of their target cells.
The years of a person’s life spent free of disease.
Biological responses to low-dose exposures to toxins or other stressors such as exercise, heat, cold, fasting, and xenohormetics. Hormetic responses are generally favorable and elicit a wide array of protective mechanisms. Examples of xenohormetic substances include plant polyphenols – molecules that plants produce in response to stress. Some evidence suggests plant polyphenols may have longevity-conferring effects when consumed in the diet.
The enlargement of an organ or tissue caused by an increase in the reproduction rate of its cells, often as an initial stage in the development of cancer.
A type of reactive oxygen species (ROS) that is generated through the activation of white bloods cells, usually in response to a viral or bacterial invader, but also as a consequence of general inflammation. Hypochlorite and other ROS can damage lipids, proteins, and DNA.
The gradual deterioration of the immune system brought on by natural age advancement. Immunosenescence is considered the most important reason for the increased rate of infections (and cancers) in older adults and is believed to be the diminished or exhausted function of the immune system that naturally occurs with aging.
A component of the innate immune system. The inflammasome is expressed in the myeloid cells and promotes the maturation of the pro-inflammatory cytokines IL-1B and IL-18. It is responsible for activation of inflammatory processes.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A peptide hormone secreted by the beta cells of the pancreatic islets cells. Insulin maintains normal blood glucose levels by facilitating the uptake of glucose into cells; regulating carbohydrate, lipid, and protein metabolism; and promoting cell division and growth. Insulin resistance, a characteristic of type 2 diabetes, is a condition in which normal insulin levels do not produce a biological response, which can lead to high blood glucose levels.
A pro-inflammatory cytokine that plays an important role as a mediator of fever and the acute-phase response. IL-6 is rapidly induced in the context of infection, autoimmunity, or cancer and is produced by almost all stromal and immune cells. Many central homeostatic processes and immunological processes are influenced by IL-6, including the acute-phase response, glucose metabolism, hematopoiesis, regulation of the neuroendocrine system, hyperthermia, fatigue, and loss of appetite. IL-6 also plays a role as an anti-inflammatory cytokine through inhibition of TNF-alpha and IL-1 and activation of IL-1ra and IL-10.
A type of white blood cell. Leukocytes are involved in protecting the body against foreign substances, microbes, and infectious diseases. They are produced or stored in various locations throughout the body, including the thymus, spleen, lymph nodes, and bone marrow, and comprise approximately 1 percent of the total blood volume in a healthy adult. Leukocytes are distinguished from other blood cells by the fact that they retain their nuclei. A cycle of prolonged fasting has been shown in animal research to reduce the number of white blood cells by nearly one-third, a phenomenon that is then fully reversed after refeeding.[1]
- ^ Cheng CW; Adams GB; Perin L; Wei M; Zhou X; Lam BS, et al. (2014). Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 6.
A family of eicosanoid inflammatory mediators produced by leukocytes (a type of white blood cell).
Independent of reactive oxidative species, mitochondria become ineffective and compromise energy supply to cells and local tissue as humans get older.
A generic term for the class of oxidative enzymes that produce bioluminescence. Luciferase is distinct from photoproteins, which produce the light-emitting reactions seen in bioluminescent organisms. The name is derived from the Latin word lucifer (lightbringer).
A medical condition that may result in blurred or no vision in the center of the visual field. A combination of genetics and environmental factors that cause oxidative stress, such as smoking and obesity, play a role. Often referred to as “age-related macular degeneration.”
An enzyme that participates in genetic pathways that sense amino acid concentrations and regulate cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription. mTOR integrates other pathways including insulin, growth factors (such as IGF-1), and amino acids. It plays key roles in mammalian metabolism and physiology, with important roles in the function of tissues including liver, muscle, white and brown adipose tissue, and the brain. It is dysregulated in many human diseases, such as diabetes, obesity, depression, and certain cancers. mTOR has two subunits, mTORC1 and mTORC2. Also referred to as “mammalian” target of rapamycin.
Rapamycin, the drug for which this pathway is named (and the anti-aging properties of which are the subject of many studies), was discovered in the 1970s and is used as an immunosuppressant in organ donor recipients.
A type of skin cancer. Melanomas typically form in the melanocytes, the pigment-producing cells located in the basal layer of the epidermis (skin). Melanomas commonly metastasize (spread) to other parts of the body. They account for approximately 10,000 deaths in the US each year.
A series of connective tissue membranes that surround the brain and spinal cord. The meninges are separated by cerebrospinal fluid, which acts as a cushion, supporting the brain and protecting it from damage that might be caused by movement or trauma.
Cancer that has spread from the part of the body where it started to other parts of the body. When cancer cells break away from a tumor, they can travel to other areas of the body through the bloodstream or the lymph system.
Tiny organelles inside cells that produce energy in the presence of oxygen. Mitochondria are referred to as the "powerhouses of the cell" because of their role in the production of ATP (adenosine triphosphate). Mitochondria are continuously undergoing a process of self-renewal known as mitophagy in order to repair damage that occurs during their energy-generating activities.
The selective degradation of mitochondria by autophagy. It often occurs in defective mitochondria following damage or stress. Mitophagy is key in keeping the cell healthy. It promotes turnover of mitochondria and prevents accumulation of dysfunctional mitochondria, which can lead to cellular degeneration.
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells used to transfer chemical energy from a food source to the electron transport chain. It exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively. NAD levels rise during a fasting state and activates the SIRT1 pathway. NADH levels rise during the fed state and serve as reducing equivalents to produce ATP.
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells used to transfer chemical energy from a food source to the electron transport chain. It exists in two forms, an oxidized and reduced form abbreviated as NAD+ and NADH respectively. NAD levels rise during a fasting state and activates the SIRT1 pathway. NADH levels rise during the fed state and serve as reducing equivalents to produce ATP.
A coenzyme that is required for the production of energy in cells. NAD+ is synthesized from three major precursors: tryptophan, nicotinic acid (vitamin B3), and nicotinamide. It regulates the activity of several key enzymes including those involved in metabolism and repairing DNA damage. NAD+ levels rise during a fasted state. A group of enzymes called sirtuins, which are a type of histone deacetylase, use NAD+ to remove acetyl groups from proteins and are important mediators for the effects of fasting, caloric restriction, and the effects of the plant compound resveratrol, a so-called caloric restriction mimetic.
A small molecule synthesized by nitric oxide synthase from the amino acid L-arginine. Nitric oxide is a highly reactive signaling molecule that freely diffuses across cell membranes. It regulates a variety of biological functions throughout the body, including vascular tone and blood flow; leukocyte adhesion and platelet aggregation; and mitochondrial oxygen consumption. Abnormalities in vascular nitric oxide production and transport are associated with hypertension, atherosclerosis, diabetes, hypercholesterolemia, and hypertension.
One of four nitrogen-containing molecules that comprise DNA. A nucleotide consists of one of four chemicals, called a “base,” plus one molecule of sugar and one molecule of phosphoric acid. Nucleotides are typically identified by the first letter of their base names: adenine (A), cytosine (C), guanine (G), and thymine (T). They form specific pairs (A with T, and G with C), and their bonds provide the helical structure of the DNA strand.
Highly reactive molecules that have the ability to oxidize other molecules and cause them to lose electrons. Common oxidants are oxygen, hydrogen peroxide, and superoxide anion.
A result of oxidative metabolism, which causes damage to DNA, lipids, proteins, mitochondria, and the cell. Oxidative stress occurs through the process of oxidative phosphorylation (the generation of energy) in mitochondria. It can also result from the generation of hypochlorite during immune activation.
Also known as TP53, this gene homolog is crucial in multicellular organisms, where it prevents cancer formation, thereby functioning as a tumor suppressor. As such, p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation. Hence, TP53 is classified as a tumor suppressor gene.
In general, anything that can produce disease. Typically, the term is used to describe an infectious agent such as a virus, bacterium, prion, fungus, or other microorganism.
The observable physical characteristics of an organism. Phenotype traits include height, weight, metabolic profile, and disease state. An individual’s phenotype is determined by both genetic and environmental factors.
A type of intermittent fasting that exceeds 48 hours. During prolonged periods of fasting, liver glycogen stores are fully depleted. To fuel the brain, the body relies on gluconeogenesis – a metabolic process that produces glucose from ketones, glycerol, and amino acids – to generate approximately 80 grams per day of glucose [1]. Depending on body weight and composition, humans can survive 30 or more days without any food. Prolonged fasting is commonly used in the clinical setting.
[1] Longo, Valter D., and Mark P. Mattson. "Fasting: molecular mechanisms and clinical applications." Cell metabolism 19.2 (2014): 181-192.
A group of lipid-signaling molecules that have diverse hormone-like effects. Prostaglandins play roles in inflammation, vasoconstriction or vasodilation, aggregation or disaggregation of platelets, calcium movement, cell growth, and thermoregulation. Prostaglandins are produced in many places throughout the human body.
Any enzyme that breaks down a protein into smaller subunits. Proteases can be found in Animalia, Plantae, Fungi, Bacteria, Archaea, or viruses.
A compound initially developed as an antifungal agent. This use was abandoned, however, when it was discovered to have potent immunosuppressive and antiproliferative properties due to its ability to inhibit one of the complexes of mTOR (mTORC1). Rapamycin has since shown interesting lifespan extension properties in animals.
Oxygen-containing chemically-reactive molecules generated by oxidative phosphorylation and immune activation. ROS can damage cellular components, including lipids, proteins, mitochondria, and DNA. Examples of ROS include: peroxides, superoxide, hydroxyl radical, and singlet oxygen.
A related byproduct, reactive nitrogen species, is also produced naturally by the immune system. Examples of RNS include nitric oxide, peroxynitrite, and nitrogen dioxide.
The two species are often collectively referred to as ROS/RNS. Preventing and efficiently repairing damage from ROS (oxidative stress) and RNS (nitrosative stress) are among the key challenges our cells face in their fight against diseases of aging, including cancer.
A polyphenolic compound produced in plants in response to injury or pathogenic attack from bacteria or fungi. Resveratrol exerts a diverse array of biological effects, including antitumor, antioxidant, antiviral, and hormonal activities. It activates sirtuin 1 (SIRT1), an enzyme that deacetylates proteins and contributes to cellular regulation (including autophagy). Dietary sources of resveratrol include grapes, blueberries, raspberries, and mulberries.
Resveratrol Autophagy ↑ Deacetylases (especially SIRT1) → ↓ Protein Acetylation → Autophagy
The loss of skeletal muscle tissue with age. Sarcopenia is one of the most important causes of functional decline and loss of independence in older adults.
Senescence is a response to stress in which damaged cells suspend normal growth and metabolism. While senescence is vital for embryonic development, wound healing, and cancer immunity, accumulation of senescent cells causes increases inflammation and participates in the phenotype of aging.
Refers to small molecules that can selectively induce death of senescent cells. From the words "senescence" (the condition or process of deterioration with age) and "lytic" (destroying).
A polyamine (an organic compound having more than two amino groups) named for having been isolated in semen. Spermidine has since been found in a variety of different tissue types, as well as foods. It is best known for its role as a potential autophagy and longevity promoter with its effects having been demonstrated in yeast, flies, worms, and human immune cells.[1]
↓ Acetyltransferase activity (especially EP300) → ↓ cytosolic Acetyl CoA → Autophagy
↓ mitochondrial transmembrane potential → ↑ ubiquitination → mitophagy (preferentially targeted)
- ^ Hartl, Regina; Megalou, Evgenia; Laun, Peter; Heeren, Gino; Breitenbach, Michael; Grubeck-Loebenstein, Beatrix, et al. (2009). Induction Of Autophagy By Spermidine Promotes Longevity Nature 11, 11.
A cell that has the potential to develop into different types of cells in the body. Stem cells are undifferentiated, so they cannot do specific functions in the body. Instead, they have the potential to become specialized cells, such as muscle cells, blood cells, and brain cells. As such, they serve as a repair system for the body. Stem cells can divide and renew themselves over a long time. In 2006, scientists reverted somatic cells into stem cells by introducing Oct4, Sox2, Klf4, and cMyc (OSKM), known as Yamanaka factors.[1]
- ^ Takahashi, Kazutoshi; Yamanaka, Shinya (2006). Induction Of Pluripotent Stem Cells From Mouse Embryonic And Adult Fibroblast Cultures By Defined Factors Cell 126, 4.
Randomly determined; having a random probability distribution or pattern that may be analyzed statistically but may not be predicted precisely.
Distinctive structures comprised of short, repetitive sequences of DNA located on the ends of chromosomes. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division. When the telomeres on a cell’s chromosomes get too short, the chromosome reaches a “critical length,” and the cell stops dividing (senescence) or dies (apoptosis). Telomeres are replenished by the enzyme telomerase, a reverse transcriptase.
An animal that has had its genome altered through the use of genetic engineering techniques, usually during early embryonic stages. Transgenic techniques are routinely used to introduce human disease genes or other genes of interest into strains of laboratory animals in order to study the function or pathology involved with a particular gene.
A fat-soluble vitamin stored in the liver and fatty tissues. Vitamin D plays key roles in several physiological processes, such as the regulation of blood pressure, calcium homeostasis, immune function, and the regulation of cell growth. In the skin, vitamin D decreases proliferation and enhances differentiation. Vitamin D synthesis begins when 7-dehydrocholesterol, which is found primarily in the skin’s epidermal layer, reacts to ultraviolet light and converts to vitamin D. Subsequent processes convert D to calcitriol, the active form of the vitamin. Vitamin D can be obtained from dietary sources, too, such as salmon, mushrooms, and many fortified foods.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Get email updates with the latest curated healthspan research
Support our work

Every other week premium members receive a special edition newsletter that summarizes all of the latest healthspan research.