#35 Gordon Lithgow, Ph.D. on Protein Aggregation, Iron Overload & the Search for Longevity Compounds
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The BDNF Protocol Guide
An essential checklist for cognitive longevity — filled with specific exercise, heat stress, and omega-3 protocols for boosting BDNF. Enter your email, and we'll deliver it straight to your inbox.
Dr. Gordon Lithgow is a professor of gerontology at the Buck Institute for Research on Aging. The topic of conversation in this episode is especially focused on Dr. Lithgow's work with C. elegans, which a tiny nematode worm he uses to interrogate questions involving the aging process and lifespan extension.
"Of course, proteins misfold during heat shock. And, as a result, these defense systems are actually acting against the normal aging process which is also the misfolding of proteins."- Dr. Gordon Lithgow Click To Tweet
Discussed in this episode:
- The role that protein aggregation plays in the aging process and neurodegenerative diseases
- How heat stress activates genetic pathways, including heat shock proteins, in worms and humans that lower protein aggregation and extend lifespan in worms.
- How too much iron can lead to protein aggregation and how vitamin D can prevent it.
- A program, directed by Gordon, called the Caenorhabditis Intervention Testing Program which tests potential longevity compounds that may extend the lifespan of several different species of worms. This serves as a broad, early exploration that may potentially help us to find ways to enhance human longevity as well.
Learn more about Dr. Gordon Lithgow
-
Gordin Lithgow Ph. D. uses c elegans worms in order to find potential longevity compounds for humans.
-
Proteostasis is the homeostasis of proteins in the body. Failure to maintain proteostasis leads to the formation of aggregates that are found in diseases such as Alzheimer's and Parkinson's.
-
-
-
-
Life extension of c elegans and drosophila via heat stress may be translatable to humans too because humans also share heat shock proteins.
-
-
-
-
-
-
-
-
-
-
-
-
Lithgow describes his approach of testing different candidate longevity compounds on c elegans and then re-testing successful longevity compounds on mice.
-
Rhonda: Hello, friends. Today, I'm sitting here with Dr. Gordon Lithgow who is a professor at the Buck Institute for Aging. One of the cool things that I really like that Gordon does is that he actually screens various compounds, both natural compounds like vitamins and minerals and other compounds to see if they potentially could be longevity compounds. And one of the ways he does this is by looking at the effects on a tiny nematode worm called C. elegans to see if there's any effect on their lifespan. So maybe you can kind of explain what are these C. elegans and...
Gordon: Sure. C. elegans is a tiny one-millimeter-sized roundworm. It's found in rotting fruit, naturally. It's found on the backs of snails, and it's the amazing genetic system that was suggested by Sydney Brenner back in the '60s to study neurobiology and neuronal development. In the late '80s, it was adopted by Tom Johnson and Mike Klass to look at longevity. And it's a fantastic system to study aging because, well, one it's transparent. So you can actually see the tissues aging in real time day after day. But two, it lives a very short time. It lives about 15 to 20 days, and that's the big advantage because you can go through lots and lots of experiments very, very quickly, fairly economically, and therefore, you can study lots of compounds and look for things that extend lifespan.
Rhonda: Right. As opposed to, for example, people that are looking at compounds and how they affect another model for a...you know, in another model. Like, mice, for example, how long do they live?
Gordon: Well, so a mouse in a lab is living two or three years...
Rhonda: Two or three years.
Gordon: ...and it's incredibly expensive to maintain the animals and, you know, these are million-dollar experiments to study longevity in mice. Whereas with the worm, again, it's very quick and you can study lots and lots of individuals fairly economically.
Rhonda: Yeah. It's super cool. Actually, I started doing my early, early research right after I graduated from the University of California, San Diego. Before I went to graduate school, I worked in an aging lab using C. elegans.
Gordon: Okay, there you go.
Rhonda: So I'm very familiar with them as a great model for aging.
Gordon: People fall in love with them, right? I mean, they actually come in and they're a little worm but before long, they're just devoted to, you know, all the biology and all the interesting things that happen. I mean, you're looking down the microscope and you're seeing all sorts of interesting behaviors. You're seeing growth. You're seeing, learning and memory even, and then you start seeing this aging process taking over and one by one knocking back the functions that you can see. And it's really dramatic when you have a mutation or a chemical compound that stops that from happening or slows it down. And people are just kind of amazed to see down a microscope a worm that's crawling around and behaving normally when it shouldn't be, when it should be dead.
Rhonda: Totally. That's what sort of hooked me into this, basically the field of aging, was looking at these worms where you can get rid of their IGF-1, you know, growth signaling pathway and literally can make them live 100% longer. I mean, it was incredible. And to think that this is the same pathway that's conserved in humans, it's like, "Well, that seems very relevant." You know, yeah, definitely, that was the thing that got me really, really interested, was that just...and seeing it myself, seeing the experiments, right?
Gordon: Seeing it, that's right. Seeing it in the microscope is really profound.
Rhonda: So these days, you're doing a lot of work on what's known as protein homeostasis or, I guess the word would be proteostasis and how that's involved in aging. And I'm not sure most people watching or listening even have any clue why protein homeostasis plays a role in aging.
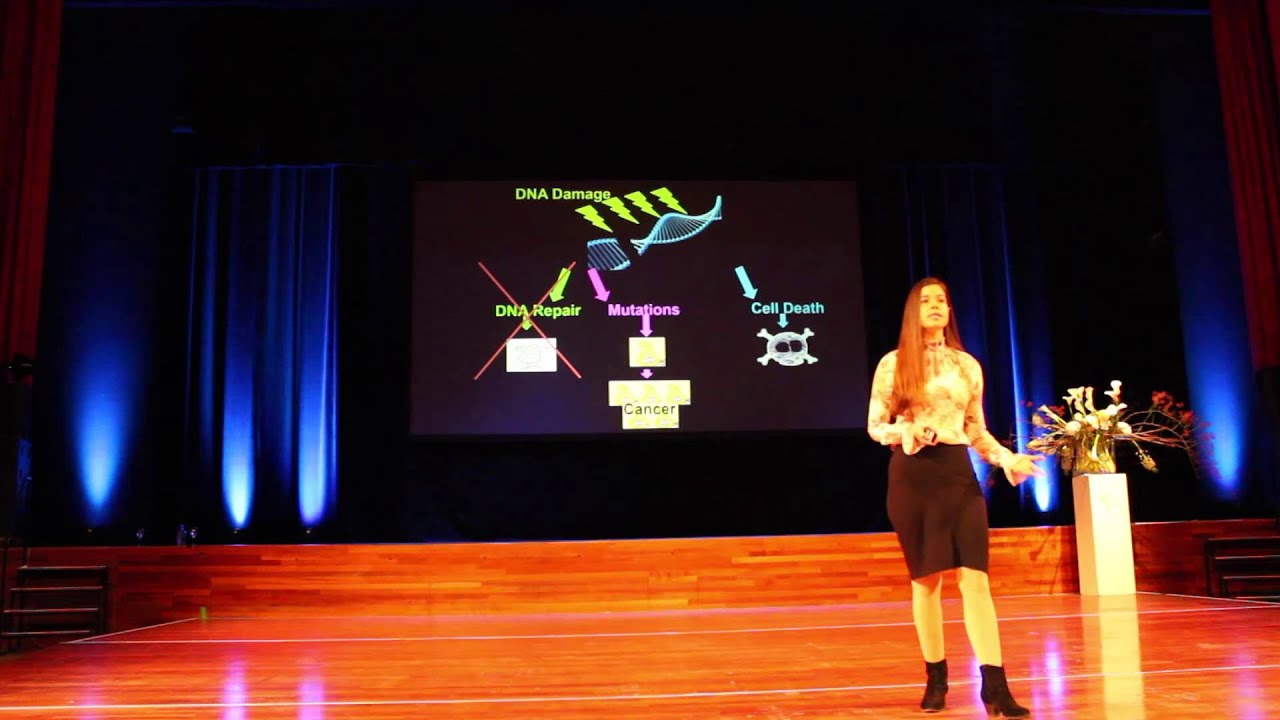
Gordon: Well, so as you know, you know, we take in our proteins and we break it down into essential amino acids and then we build our own proteins and the worm builds its own proteins. And those proteins have a three dimensional shape, and that shape's important for our function, of course. And during aging, the general observation is that the proteins lose their shape in various ways. For example, during Alzheimer's disease, the protein beta-amyloid loses its shape, undergoes various conformational changes, becomes toxic, neurotoxic, but eventually ends up as an insoluble protein in amyloid plaques in a diseased brain. Now, that process happens in Parkinson's disease as well with a different protein, alpha-synuclein. But actually, we believe it's happening to thousands of proteins. And a few years ago, we published a paper where we showed that many hundreds, if not thousands of proteins, undergo conformational change during aging and come out of solution. And it so happens that those proteins are enriched for proteins that determine lifespan.
Rhonda: So when you're saying out of solution, you're saying they're insoluble...
Gordon: They become insoluble.
Rhonda: ...can you at that point say it's an aggregate? Is that technically accurate?
Gordon: Biochemically, insolubility is related to aggregation.
Rhonda: Precipitate, right?
Gordon: Aggregation is the coming together of insoluble protein in a single [inaudible 00:04:45].
Rhonda: So you're saying these aggregations or these insoluble proteins that are happening, you're saying hundreds of proteins are happening, with age, this is happening. You know, this is partly because of damage that accumulates, correct? I mean, a lot of damage that damages DNA, so things like, you know, reactive oxygen species, inflammatory cytokines, these things are damaging our DNA are proteins as well.
Gordon: Right. I mean, proteins sustain lots of damage in the normal course of metabolism. But it's actually not too clear as why this particular set of proteins come out of solution, and that's something we're pursuing and other labs are pursuing as well. Cynthia Kenyon's lab discovered a very similar mechanism around about the same time. And so I think we're all really interested as to why these proteins come out of solution and are enriched for proteins that determine lifespan. That kind of suggests that the proteins of insolubility or misfolding or conformational change in itself has something to do with the aging process. And I think this is really important because here's a process that's been studied for decades in the context of neurological disease, Alzheimer's and Parkinson's and so on. But if the same process is happening in the course of normal aging, it shows a connection between normal aging and disease and that's something I think that lots of us are interested in right now. What are the mechanisms of normal aging that are likely to accelerate age-related pathologies and disease?
Rhonda: Right. And if this is happening in not just our neurons, if it's happening in our endothelial cells that line our blood vessels, I mean, I often think about that as another, you know, tissue that is prone to, you know, insoluble proteins forming. I'm not sure if that's actually accurate.
Gordon: No, I mean, the amyloids form in lots of tissues in our body. There's very specific diseases associated with that. But I think what we're seeing is that this amyloid formations is a more general aging process. It's just going on probably in most of our tissues, if not all, and maybe then drives disease pathology that becomes obvious to us when we look at it as a disease.
Rhonda: So it's disrupting just normal tissue function, whatever tissue that is, it's disrupting maybe mitochondrial function, just, you know, the everyday things that are happening aren't happening as well and this sort of can, you know, lead to or be part of what we call the aging process in a way.
Gordon: Absolutely. You know, and it's not to say it's the only aging process. It clearly isn't the only thing that is changing. Everything is changing. There's evidence, for example, on Alzheimer's disease that there's a metabolic problem that happens before you start seeing aggregation of proteins. So who knows how all these things interact with each other, but it's important. I think that's firmly established now that this is a major mechanism of aging.
Rhonda: Right, yes. Well, I remember, gosh, it must have been like 12 years ago, when I first read a paper of yours where I believed you may have been a post-doc because you were first author on this paper. And the paper was you had found that heat shocking worms... Actually, I think it was just at this early paper was a single heat shocking. You increased the lifespan of the worm by like 15%, and this was totally dependent on the production of something called heat shock proteins or HSPs which people have heard me talk about before. And then you published again showing that multiple heat shock treatments could increase the lifespan of the worm like even more robustly. So could you maybe talk, you know, just a little bit about, you know, how heat shock is this type of hormetic stress and how this can have beneficial effects.
Gordon: Sure. I remember when I saw this for the first time as a post-doc. and I ran into the office of my supervisor, Tom Johnson, and I said, "Look at this. This is amazing. You stress the animals and they live longer. How is that possible?" And he said, "All right, you've just discovered something that John Maynard Smith published in "Nature" in 1950s." And he pulled this paper, you know, out of his drawer, and sure enough that John Maynard Smith, the evolutionary biologist, had been looking at tradeoffs between reproduction and lifespan and he had stressed, in this case, flies, he stressed the flies with a heat shock and they had lived longer. And it was kind of amazing that this was in the 1950s and this was before our understanding of molecular chaperones and stress responses. And so they probably could never put that discovery into the context of the molecular and cellular processes that were going on. And what was going on was the animals were being stressed, they're ramping up their defenses against misfolded protein. Of course, proteins misfold during the heat shock. And as a result, these defense systems are actually acting against the normal aging process which is also the misfolding of proteins. So it kind of makes sense to us now that these so called hormetic responses are the production of molecular chaperones, also ramping up of autophagy, the process of breaking down proteins. And a beautiful paper from Malene Hansen a few weeks ago was showing that...
Rhonda: Yeah, I saw that.
Gordon: ...autophagy is critical for this response to heat shock. So it's nice that things have come full circle and we've got a better understanding of what's going on there.
Rhonda: It totally makes sense, though. I mean, if you think about it, like you said, you know, in the case of heat, there's lots of examples of hormetic stressors. I mean, heat's one, there's fasting. There's a lot of xenobiotic, you know, compounds or xenohormetic compounds, like curcumin, these sort of things can...you know, they're slightly toxic in a small dose. And because of that small dose, I think dose is important, it activates, like you said, all these cellular stress response pathways that then help us deal with stress better. And guess what, aging is a stress. So, you know, you're not only increasing things that help proteins keep their three-dimensional structure, but you're increasing antioxidant pathways and anti-inflammatory, just a whole host of things and autophagy, wanting to get rid of or clear away damaged proteins, damaged cells. And it's so funny. I hadn't thought about heat shock increasing autophagy before and I was doing some background reading to sort of prepare to talk to you and I came across that paper and I was like, "Oh, this is awesome," you know, because I hadn't thought about it.
Gordon: Yeah, no, Malene told me about this before the paper was published, she was sitting in my office telling me about this and I thought, "Ah, this is fantastic." You know, here's a mechanism now that really explains why a stress is actually leading to longer life.
Rhonda: Yeah, and it makes perfect sense. Things like heat stress would increase, you know, the activity of machinery that we have to degrade proteins that are damaged like the proteasome. And it would make sense that autophagy, which is another pathway to do that, would also be also be part of that stress response pathway as well. But what's really cool is that this is very relevant for humans, right? Because...
Gordon: Yeah.
Rhonda: ...humans have heat shock proteins. And our heat shock proteins are also responsive to heat.
Gordon: Absolutely.
Rhonda: So, I mean, this isn't like just understanding worms. It's something that's, you know, being translated to humans.
Gordon: And actually, there's a body of literature about pre-stressing organs ahead of surgery that's very interesting and might be relevant to this, where, you know, there's just better recovery from surgery if there's been a pre-stress. And similar literature on starvation or fasting ahead of various treatments for cancer, for example...
Rhonda: Chemo...
Gordon: Chemo, yeah. So we might not know all the details there, but absolutely, I think this is really important. It's probably a neglected area actually. I think that, you know, Malene's papers brought it back to light, that these stresses are anti-aging and potentially beneficial and we need to think about how you would modify the stress in itself for humans. Obviously, we don't want to stress people, stress is damage, no doubt about it.
Rhonda: Right. Too much is, yeah.
Gordon: But can we harness that endogenous machinery that counteracts the stress? And I actually think that's what we're doing with a lot of the chemical compounds that we discover extend lifespan, is that they are either hitting pathways that regulate stress responses or they are providing a sort of, we call...damn, I forget what we call it. Sorry. I think some of the chemical components that we discover that extend life span are actually doing this. They're harnessing the endogenous stress responses.
Rhonda: Absolutely, absolutely.
Gordon: They're either activating the regulators of stress responses or they're causing segmental stress. So you're seeing a limited stress response or only parts of the stress response are being activated but that's enough to give you beneficial effects.
- Rhonda: Mm-hmm. Yeah. So I totally wanted to mention something before you jumped into the compounds that was related to the heat stress, and that is, there was a study, and you may be interested in this, there was a study I read. It was published not too long ago, just a few years ago, where people were looking at the heat shock response in humans that were sitting in a sauna. So, of course, that would be, you know, a way that humans can activate their heat shock proteins. So humans that sat in 163 degree Fahrenheit sauna for about 30 minutes increased their heat shock proteins, including Hsp70 by 50% over baseline. And that was actually sustained for about 48 hours. So that elevation stayed for around 48 hours, which is really cool because it's kind of like a take-home, well, maybe we can activate our heat shock proteins from the sauna. And to sort of go one step further, because I've been sort of obsessed with heat shocking in sauna for probably since I started doing research many, many years ago and I was working on heat shock a little bit and HSF-1 and all this stuff. But I recently went to Finland last November. And there's a researcher there. His name is, Jari Laukkanen. And he's been doing...he's a...MD, PhD. He is a cardiologist, so a lot of his focus has been on heart health. And the sauna is something that is ubiquitous in Finland. I mean, everyone has a sauna, everyone. It's just ridiculous. So he does a lot of research on sauna and he published a couple of studies that one came out in 2015 where he was looking at all-cause mortality in sauna use. So men, this was like 2,000-men cohort, and men that had used the sauna like 2 to 3 times a week had a 23% or 24% lower all-cause mortality.
Gordon: Wow.
Rhonda: Men that used it 4 to 7 times a week had a 40% lower all-cause mortality compared to men only used it once a week. So it was like a dose response effect.
Gordon: Incredible. Yeah.
Rhonda: But there's where you'll be interested. So he just published this paper last December, same cohort of men. But this time he was looking at Alzheimer's disease. And what he found is that men that use the sauna 2, 3 times a week had a 20% lower risk of getting Alzheimer's. If they used it 4 to 7 times a week, they had a 60% reduction in Alzheimer's disease risk, which is really kind of cool because it goes with your molecular mechanistic work in lower organisms on, you know, the protein aggregation and the heat shock and the stress response pathway.
Gordon: Yeah, no, I spent most of my career just interested in worms. I mean, I thought if we can solve worm aging, that's fantastic. But over the years, this creeping realization that actually what we're doing could be important for people as well. Making all these connections to disease and all the model organism people coming to this conclusion, working in flies and mice and yeast even that these are basic mechanisms of aging. They're likely to be playing out in humans as well and therefore we should do something with this knowledge we have accumulated over the last 25 years. It's really time to try and translate that and do something that might be beneficial.
Rhonda: Yes. Another thing that you've done I think is very relevant is your work on iron. So you had looked at how excess dietary iron affects, again, I think protein homeostasis in worms?
Gordon: Absolutely, yeah. Yeah. Well this was inspired by my wife, Julia Anderson's work on Parkinson's disease where many years ago, she published a series of papers showing how important iron was causing damage to complex I in the mitochondria through redox reactions, and then that very specific damage would play all the way to neuronal death, the dopaminergic neurons. So we went back and looked to iron. Basically, we had a collaborator, David Killilea, who was able to show that iron levels become elevated during normal aging in the worm. We thought, "Well, that's interesting because that's what happens in human brains and other tissues." And so we did a couple of things. First of all, we fed exogenous iron, so we increased the iron levels in the media, in the diet that accelerated aging, so it shortened lifespan, but it also accelerated the accumulation of insoluble proteins. So it accelerated this sort of molecular pathology of aging. And the other thing we did was to feed the worms on a chelator, an iron chelator. And in that case, the iron levels did not rise during aging and the worms lived longer and they protected against protein aggregation as well.
Rhonda: Oh, wow. That was going to be my next question, actually.
Gordon: Yeah. So all sorta made sense.
Rhonda: Right. So the iron that you're feeding these worms, is there any way that it can be physiologically relevant to humans, like, the levels that you were giving them possibly?
Gordon: I believe so. I think the experiments in mice with Parkinson's disease, for example, those are right about the levels that you could be exposed to, especially if you're working with metals, you're a welder or, you know, certain careers like that. And the epidemiology suggests that indeed that leads to increased risks of Parkinson's disease. So I think coming back to normal aging, I wouldn't be surprised if these kinds of levels of iron are important. The...
Rhonda: Are you familiar with the...there's a few gene polymorphisms and one is in the hemochromatosis gene and people can get hemochromatosis where they're absorbing way too much dietary iron. So that seems like it could be something very relevant if someone is homozygous for those polymorphisms in that gene.
Gordon: It could be and it'd be interesting to look at their aging characteristics and ask whether there's any sort of accelerated aging phenotype there.
Rhonda: I know there's all sorts of problems, so I wouldn't be surprised if there was. What's so funny is I didn't think about you're mentioning Parkinson's and how Parkinson's is associated with iron accumulation and in the mitochondria or damaging mitochondria and this is leading to death of dopaminergic neurons. But what I was familiar with was Alzheimer's disease and how there's...I know there's especially a cluster of polymorphisms. One is in the hemochromatosis gene, the other one's in the transferrin gene which binds free iron together if you have, like, this right combination. I don't know how frequent it is in the human population it occurs, people actually have a five times increased risk of Alzheimer's disease. So there's definitely a connection between iron and, obviously, neurological diseases in general.
Gordon: And other metals as well.
Rhonda: Other metals as well. Yeah, you've looked at some other metals, right? Like copper?
Gordon: Yeah, and copper and manganese. And I think copper is probably critical in Alzheimer's disease. And again, this is possibly an underappreciated aspect of neurological disease partly because I think that it's difficult to think of ways to go after that as a target. You know, so if you say, you know, "You know, we're going to manipulate the levels of metals." Well, you know, a third or a half of all proteins have metals as part of their active sites. And the idea of treating a metal disorder is a bit...little bit difficult to get your head around and there are very few pharmaceutical companies in the world who really go after metals. But, you know, I don't see why we shouldn't be asking the question, ‘can we modulate metals? Can we modulate the activity of metal transporters in particular tissues? And can we prevent the elevation of metals in tissues during aging?’ That basic prevention of elevated levels of metals could be enough to protect us against disease.
Rhonda: Yeah, absolutely. I think it's a grand point question. You know, people are supplementing with all sorts of vitamins and minerals and some people are taking way too many. You know, iron is something that...I think, you know, people should get their iron levels measured. They shouldn't just be blindly taking an iron supplement, I mean, because that could be completely dangerous.
Gordon: That's right. Absolutely.
Rhonda: And, you know, with these gene polymorphisms, like, it's sort of a pet topic of mine, so I'm sorry if I'm going off on this. But, we've sort of evolved in different regions across the globe and there's different food availability, different minerals in the soil, things like that. And so we sort of, like, depending on, at least this is the thought, depending on how much minerals were in the soil, what type of food we had available, we sort of, acquired certain mutations that became more frequent in the population that became polymorphism. So it may be some people... And you can see this when you look, that if you look at, you know, a lot of different gene polymorphisms, a lot of them do have to do with minerals. They have to do with vitamin intake. And so all these things are sort of different in people. And so I think it is very relevant to look at how this affects aging, you know, for multiple reasons. So I'm actually glad that someone is doing it. Of course, the pharmaceutical drugs are always thinking of it from a different angle and that may not be the way, but it's all very important. But sort of to kind of go on the next topic, you've also looked at vitamins. And I was very excited about your most recent, not most recent, but a recent publication of yours that had to do with one of my favorite vitamins, which is not actually a vitamin. It actually is a hormone.
Gordon: A hormone, yeah.
Rhonda: But vitamin D. So you'd found some really interesting findings with vitamin D.
Gordon: Yeah. We were looking at a C. elegan strain that was engineered to express A-beta, so human A-beta. Now, when you do this in the worm, you see these tiny aggregates form, like mini plaques. And something about that is toxic and the animals become paralyzed.
Rhonda: So this is in their muscle cells.
Gordon: It's in their muscle cells, although you can do the same in neurons as well.
Rhonda: In whatever, okay.
Gordon: But it always makes the worm sick. So this was a strain created by Chris Lincoln in Colorado. And we were looking for chemical compounds that suppressed the paralysis. So very easy, you look down the microscope, if the worms are still crawling around after you've turned on the expression of the A-beta, you've got something that's protecting against the aggregation process. And Carla Mark [SP], post-doc in the lab, conducted a screen on natural products. We look at all sorts of different ligands. This happened to be a natural product ligand. And came to the office and said, "Hey, Vitamin D." And we said, "Well, we're not going to work on that because there's a paper every day on vitamin D. And surely people have been studying vitamin D for decades. There's nothing new to learn about vitamin D." But the thing that intrigued us was when we started to look at the epidemiological literature and realized that when you're deficient, when humans are deficient of vitamin D, they're an elevated risk for many adult cancers but also neurological disease. And if you look at the spectrum of diseases it almost looks like many of the chronic diseases of late life. So I thought, "This can't be. Is this possible that vitamin D deficiency is really some sort of accelerated aging? Is that's what going on here in people?" So we thought we may as well follow this, because there's not a lot in the literature about A-beta and protein conformation and aggregation and so on. So we did many, many other experiments and found that indeed vitamin D had an effect, widespread effect on the proteome. So it was preventing protein insolubility as age-related rise in protein solubility across hundreds of different kinds of proteins and different organelles in the cell and different issues.
Rhonda: So it was essentially helping the proteins age better.
Gordon: Exactly, yeah, suppressing this phenotype of aging. And then we combined treatment with compounds or feeding with vitamin D in this case with different genetic backgrounds. And so we discovered that there's a requirement for certain genes, transcription factors, and these are transcription factors that normally respond to stress and they were back to stress. And it seems like vitamin D somehow is able to elicit the endogenous defense system, detoxification systems. So it's turning on those systems.
Rhonda: Like, Nrf2 regulated?
Gordon: Right, that's right. Yeah, the oxidative stress transcription factor, Nrf2. It requires Nrf2 in the worm to see the beneficial effects on the proteome. How weird? So we're following that up. But all this was new for vitamin D. We began to show this data to some clinicians, experts in vitamin D metabolism in bone and so on. And they were really excited by this, which is always nice when you study worms, suddenly you've got a clinician excited. And I think they're excited because at the heart of it, you could see how an effect on a global process like protein aggregation which is associated with lots of different diseases could explain perhaps why vitamin D deficiency is associated with neurological disease and other diseases. So it almost like was giving them a handle on a potential mechanism that might explain a lot for what's going on in the epidemiological data. And we thought it was cool because when we talk about vitamin D to your colleagues or friends or the general public, there's a response. People are interested in it. It's cheap, it's readily available, many people are already thinking about it in their own health. So it makes a connection between, you know, the curiosity research we do in worms and actually people's lives. So we're enjoying working on it.
Rhonda: Very cool. Well, I have a thousand questions about that. So, I mean, obviously, the question you probably get from every single person that you talk to about this research and that is, okay, so do the worms make vitamin D? We make vitamin D in our skin from UVB radiation.
Gordon: Correct.
Rhonda: And I always thought of the worms as being soil-dwelling but you mentioned at the beginning of the podcast that they actually are on fruit. So potentially, they're exposed.
Gordon: They probably are exposed to light. Now, worms will run away from light. If you turn the UV light on in a microscope, they'll move. But they probably are exposed to a certain amount of light. Now, do they make it in the wild? We don't know. What we do know is that if we feed D3 which is the metabolite you buy at the drug store. D3 is converted into 1,25-dihydroxyvitamin D. So there's two steps. One in the kidney, one in the liver in mammals that does that conversion. Now, we know if we feed the worms D3, they're able to make the 1,25 vitamin D. So we think that there's conserved metabolism between mammals and worms that suggests that maybe the worms are really making it in the wild as well. They've got the apparatus. They've got the enzymes. We also know that they have the precursor to vitamin D that we have in our skin that we turn into D3. They've got tons of that in their tissues normally. We actually feed the worm's cholesterol as part of their normal diet.
Rhonda: Really? Oh, they have 7-hydroxycholesterol.
Gordon: They do. They have 7-hydroxycholesterol in large amounts. So our expectation is, and we'd never really been able to think how to do this experimentally, but if we took worms from the lab and took them outside into the sunlight, we might be able to detect them making 1,25.
Rhonda: Well, why don't you just... There's a couple of experiments I would think about is, one, shining UVB on them, right, and seeing if that works, because that's how we do it. So UVB radiation hits the 7-hydroxycholesterol, and then that's converted by D3, that gets transported into the bloodstream, then two hydroxylation steps later, you get the active hormone.
Gordon: Right.
Rhonda: So that could be one thing you could do. The other thing is did you guys try feeding 1,25-dihydroxyvitamin D to the worms?
Gordon: We did. Actually, we fed a whole bunch of different metabolites of vitamin D. And basically, any metabolite that can be converted into the active form is beneficial.
Rhonda: Okay, cool. So that sort of makes sense.
Gordon: It makes sense.
Rhonda: Yeah. You'll be interested to know, if you haven't already seen this literature, because I also have published on vitamin D and very obsessed with it. Are you familiar with the work that was published, geez, it must have been like 10 years ago now, but on the vitamin D receptor knockout index.
Gordon: Yes, yes, yes, yes.
Rhonda: Have you seen those animals?
Gordon: I've never seen them personally, but I've certainly read about them.
Rhonda: Seen pictures of them? Well, at four months of age, they're both...you know, if you have normal wild type mouse and you knock out the vitamin D receptor, then you look four months, they look the same age. And then at eight months, that mouse looks like a progeria. I mean, it doesn't even look like a two and a half year old mouse would normally look. It looks like some sort of progeria kind of phenotype where there's no hair, the skin is all wrinkled, their organs are all, like, shutting down. I mean, it seems like a very progeria type of phenotype. I don't know if that's...
Gordon: Certainly, the paper suggests that and there are specific measures that are absolutely in keeping thermal aging. There's probably a lot of other things going on as well, I imagine, that when you really seriously mess up vitamin D metabolism, it's going to have lots of effects.
Rhonda: Yeah, that's a given.
Gordon: Yeah. But absolutely, very exciting when we went back and looked at those papers and thought, "Oh, this could make sense."
Rhonda: Right. And there's also, like, if you look at the epidemiology stages in humans, we know that, for example, there was a very large meta-analysis, like, 33 different studies that were looked at ranging from, you know, the year 1966 all the way to 2013, so broad range of time, looking at people's blood levels of 25-hydroxyvitamin D which is the precursor and is the most stable form. And what the meta-analysis found is that people that had blood levels between 40 and 60 nanograms per milliliter had the lowest all-cause mortality compared to those that, you know, had lower vitamin D levels or even really, really high ones. But there's some randomized controlled trials that have looked at giving people high dose vitamin D supplementation, like 4,000 IUs a day and it improved cognition, whereas low dose, 400 IUs a day would be considered low dose, that did not...had no effect. So I think there is certainly a lot of evidence associative studies and also there's some randomized-control trials that really does point to the fact that vitamin D is regulating the aging process. It's been shown to regulate telomere length. I think that it's absolutely probably regulating the aging process and this whole protein aggregation angle is new to me. I didn't know that it really played a role in that and that's super cool because I do know that protein aggregation plays a role in the aging process. So it kind of, you know...
Gordon: Yes, it's beginning to join up in a sensible story. Now that said, we should be cautious. And I'm not an MD and I am not prescribing vitamin D for anyone, although it's likely that if you are deficient, you really would benefit from coming up into a sensible range. At high doses, and these are usually very high doses as a result of some accident, but obviously it causes mineralization and that can be serious. And so, you know, I think it's up to people to talk to their physicians about it, to get tested perhaps for levels. It's probably almost completely harmless to be taking an additional 1,000 units a day on top of whatever is in your diet, but really talk to your doctor about it.
Rhonda: I think that's excellent advice and, you know, most people don't get their vitamin D levels measured and I think that's exactly what people should do. You know, they should get their D levels measured before and after supplementation. Both. I mean, it's not a hard test to do. If you have health insurance, it's covered. If you don't, it's still really cheap. I've done it. You know, it's not an expensive test and it's very worth it, so I agree that it's very important to get your levels measured and you don't want to just blindly start taking like 20,000 IUs.
Gordon: Yeah. Especially if you're a C. elegans.
Rhonda: Okay. So the last thing I want to talk to you about that is really super cool that you're doing, is you're involved...I know that the NIH set up this program that was like this intervention testing program in mice, right?
Gordon: That's right.
Rhonda: And they were looking at compounds that could potentially affect longevity in mice. But in parallel, you are doing something really cool in worms.
Gordon: Yeah. I mean, the mouse program has been very successful. Rapamycin was the drug that emerged as being robust and reproducible at all three sites where these experiments were conducted in mice. But a few years later, I guess it was a feeling that maybe we can accelerate this if we utilize the speed of the worm and also utilize the great genetic diversity in Caenorhabditis itself. Caenorhabditis is found all over the world, has incredibly large genetic diversity, and the thinking there is that, well, if you can find compounds that work in not only different labs to the same extent, but also at different genetic backgrounds, then you've maybe got a high value candidate to then take forward into more expensive mouse study. So, you know, it made perfect sense to us. We were very fortunate to be funded with Monica Driscoll and Patrick Phillips, the other two investigators involved in the study. It's a large study with lots of people in all of our labs.
And we sat down for the first time to talk about doing worm aging experiments and to work out how we were going to do this so that we're all doing the same thing. We were horrified, absolutely horrified to see the differences between labs and protocols. It used to be the simplest experiments in the world. You take worms, you put them on an agar plate, you squirt a compound on there, you watch them every day until they all die. It's the simplest thing in biology, except we were all making our plates differently, growing our bacterial food differently, handling our worms differently, everything was different.
So it actually took us over a year to standardize the protocols and start to see similar results even without treatment, just growing worms and measuring aging. It took us about a year to really get that done. But then we had this wonderful platform where we could go in and say, "Well, let's test some of the compounds that were already out there and published and let's test some compounds that have been looked at in mouse studies and ask are they robust and reproducible?" And the results are mixed, to be honest. We do. We are able to find reproducible and robust compounds that work in lots of different genetic backgrounds and extend lifespan in all three labs. But we find also a great degree of variation and different kinds of variation that we'd never imagined before. So I think it has proven to us that this is difficult stuff, doing this, and we really need to pay great attention to the protocols that we're using and be able to communicate those and convey them to all of the laboratories if we expect other people to be able to reproduce our findings.
Rhonda: Yeah. And what sort of compounds are you... So there's like a top 10 compounds that you were looking at?
Gordon: Yeah. I mean, we selected 10, as I said, just to get started, and those included some compounds that we had published, including Thioflavin T which is a compound that binds amyloids. And we hoped and we think it does promote protein homeostasis as a result of this. It also turns on stress responses as we were talking about earlier. So Thio T was one that we felt was going to be robust. It turned out it was. It was robust and reproducible.
Rhonda: So that extended the lifespan of C. elegans and all the other...
Gordon: Yeah, C. elegans, C. briggsae and other species, C. tropicalis and other species. And there were some compounds that did really well in elegans when they were first published but actually didn't then do well in briggsae. So there could be many, many reasons for that but it was kind of what we expected that different genetic backgrounds will respond differently to a compound. And we found compounds that really didn't do anything at all under our protocol. Now that's not to say they don't work in someone else's hands in their protocol, but now I think we've got a deeper understanding of the major effects of just subtle changes in the way we do experiments. So they don't work in their hands but that does not mean they're not promising candidates. It just means we need to think about what conditions they are going to work in.
Rhonda: It seems like the compounds that are working in multiple different species are probably, you know, the best candidates, in my opinion. But, you know, the ones that are at least working in some species are also, seems... I think alpha-Ketoglutarate was another one that was in some.
Gordon: Yeah. Yeah, it's doing really well. I mean, it's great when you go to a paper and, you know, a really great paper that you like and you just are able to reproduce it. It's fantastic. So alpha-Ketoglutarate was one of those candidates. Yeah, I mean, I think we also want to investigate why compounds do not work in particular strains because that could tell us something about genetic-specific responses to compounds. And this may become important as you move towards humans where there may be particular metabolic pathways that we want to avoid engaging, or we might have genetic information on that would suggest we shouldn't be treating this group of people.
Rhonda: Right, exactly. Personalized medicine sort of, this interaction between genes and compounds and there's lots of interactions between drug metabolism and the way we metabolize drugs and, you know, the genes that we have. So that [inaudible 00:37:06] actually makes sense. But so the next step then is once you... So if you have like these compounds that seem very promising that you have identified in the various species of worms, do you communicate to the mouse community and they sort of potentially will look at those?
Gordon: Absolutely, I mean we will publish everything, positive and negative, with respect to life span effects, also healthspan, we're looking at healthspan. But obviously...
Rhonda: How do you do that in worms? So what's...
Gordon: Well, you know, that's a big debate right now is how to do that. Of course, the worms are changing their behaviors as they age, they're becoming slower, they're eating less, they cease reproduction. Eventually, they become paralyzed, essentially not moving at all in the plate. So there's plenty of things to look at. Also, their tissues are changing and you can look at the tissues themselves, and really we're just kind of sorting out what the best parameters might be right now. Resistance to stress, for example, goes down with age. And so maybe we just look at resistance to stress at multiple ages and ask if the compounds are able to maintain that resistance.
Rhonda: What about compounds that are able to really work well when you stress the animal? So let's say you have a compound that is something that is like a xenohormetic kind of compound, which you may see a very small effect on lifespan just under normal aging conditions, but what if you were to like stress them? You add some sort of oxidative stress or something and then you see a really robust, like do you think you might be missing some of those sort of compounds?
Gordon: I believe so and I think that they might come out of our next series of experiments where even where we have a negative result on lifespan, we will look at healthspan. We'll look at the stress responses and ask, "Well, is this something that's really making the animal healthier for longer," which, of course, we are interested in, but maybe just fails to increase the maximum lifespan.
Rhonda: Can I put a bid in for a compound you should look at?
Gordon: Yeah.
Rhonda: Sulforaphane. Sulforaphane is...are you familiar with sulforaphane?
Gordon: No, please tell me.
Rhonda: So sulforaphane is a xenohormetic compound. It is produced in cruciferous plants. So, you know, anything from broccoli to kale to cauliflower.
Gordon: Oh, yeah. I remember now.
Rhonda: So it is produced when the plant is, you know, crushed or broken and it comes in contact with an enzyme called myrosinase and then you produce sulforaphane. Sulforaphane is, to my knowledge, the most potent dietary...naturally occurring dietary activator of Nrf2 pathway. I've done a lot of reading about it. I've interviewed Dr. Jed Fahey, who's at Johns Hopkins who worked with Talalay who sort of discovered that it was the, you know, activator of this whole Nrf2-Keap1 pathway. So I'm sort of really familiar with the field and I was doing a lot of reading trying to figure out...you know, because in humans, there has been a lot of clinical studies in humans showing it lowers inflammation, biomarkers of inflammation in humans, it lowers biomarkers of oxidative stress in blood cells, it affects glutathione, just everything, right? And all sorts of cancer prevention studies Alzheimer's disease in animals, I mean, lots of animal studies. But I couldn't really find any lifespan studies and I was looking specifically for drosophila or c elegans or something, and I came across this paper that was published in red flour beetle or something. I'd never heard of it. Anyways, they fed these red flour beetles sulforaphane and it extended their lifespan and then they did some oxidative stress and it really robustly extended their lifespan. So I would be really interested...
Gordon: Good candidate.
Rhonda: ...to see if it does. I mean, I would bet that it does something in C. elegans. So sulforaphane. I will send you an email.
Gordon: Please do. And actually, you know, we are really interested in hearing stories like this and we want other scientists and other people to make suggestions to the consortium for testing compounds. We have another 10 that are in process right now, but this is going to continue for the next few years and we hope to get through hundreds of compounds eventually, so we are looking for suggestions.
Rhonda: Cool. It's definitely one. Yeah, I would love to see, I mean, really love to see it. So that would be super cool. Well, Gordon, I really... Thank you so much for taking some time to speak with me about your research and how you're trying to look at all these various pathways as they relate to aging and protein homeostasis and these compounds that may extend healthspan and lifespan, all very relevant to us down the line.
Gordon: Hope so.
Rhonda: So pleasure talking with you today.
Gordon: Nice to talk to you, thank you.
Rhonda: And if people want to find you, they can find you at the Buck Institute for Aging.
Gordon: Absolutely, yep.
Rhonda: And if they want to learn more about your research, you'll be there.
Gordon: That's right. Thank you very much.
A jelly-like substance, equivalent to vegan gelatin, obtained from the cell walls of algae. It has diverse uses ranging from plant biology and microbiology research to even various culinary uses. Agar itself is indigestible for many organisms so that microbial growth does not affect the gel used and it remains stable.
The death rate from all causes of death for a population in a given time period.
A protein present in the human brain, found primarily at the synapses – the junctions between neighboring neurons where the exchange of electrical signals and neuronal communication occurs. Aggregation, or clumping, of alpha-synuclein proteins is a hallmark of Parkinson's disease, a neurodegenerative disorder of the central nervous system. Hsp70, a heat shock protein, has been shown to reduce formation of alpha-synuclein oligomers and reduce associated toxicity.[1]
- ^ Hashimoto, Tadafumi; J. McLean, Pamela; Danzer, Karin M.; Ruf, Wolfgang P.; Putcha, Preeti; Joyner, Daniel, et al. (2010). Heat‐shock Protein 70 Modulates Toxic Extracellular Α‐Synuclein Oligomers And Rescues Trans‐Synaptic Toxicity The FASEB Journal 25, 1.
A neurodegenerative disorder characterized by progressive memory loss, spatial disorientation, cognitive dysfunction, and behavioral changes. The pathological hallmarks of Alzheimer's disease include amyloid-beta plaques, tau tangles, and reduced brain glucose uptake. Most cases of Alzheimer's disease do not run in families and are described as "sporadic." The primary risk factor for sporadic Alzheimer's disease is aging, with prevalence roughly doubling every five years after age 65. Roughly one-third of people aged 85 and older have Alzheimer's. The major genetic risk factor for Alzheimer's is a variant in the apolipoprotein E (APOE) gene called APOE4.
A toxic 42 amino acid peptide that aggregates and forms plaques in the brain with age. Amyloid-beta is associated with Alzheimer's disease, a progressive neurodegenerative disease that can occur in middle or old age and is the most common cause of dementia. Heat shock proteins have been shown to inhibit the early aggregation of amyloid beta 42 and reduce amyloid beta plaque toxicity [1].
A molecule that inhibits oxidative damage to DNA, proteins, and lipids in cells. Oxidative damage plays a role in the aging process, cancer, and neurodegeneration. Many vitamins and plant-based compounds are antioxidants.
An intracellular degradation system involved in the disassembly and recycling of unnecessary or dysfunctional cellular components. Autophagy participates in cell death, a process known as autophagic dell death. Prolonged fasting is a robust initiator of autophagy and may help protect against cancer and even aging by reducing the burden of abnormal cells.
The relationship between autophagy and cancer is complex, however. Autophagy may prevent the survival of pre-malignant cells, but can also be hijacked as a malignant adaptation by cancer, providing a useful means to scavenge resources needed for further growth.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A transparent nematode species (a type of roundworm), about 1mm in length. The first multicellular organism to have its whole genome sequenced. Because they have a short lifespan (about 14-15 days), they are a good model organism for aging research. Strains are inexpensive to breed and can be frozen. When subsequently thawed, they remain viable, allowing long-term storage.
A protein that can bond to free metals. Often the metal atom is used to lower activation energy for reactions and speed up reactions in the body. Common examples of chelators include porphyrin rings in hemoglobin and chlorophyll.
A waxy lipid produced primarily in the liver and intestines. Cholesterol can be synthesized endogenously and is present in all the body's cells, where it participates in many physiological functions, including fat metabolism, hormone production, vitamin D synthesis, and cell membrane integrity. Dietary sources of cholesterol include egg yolks, meat, and cheese.
The first enzyme in the electron transport chain. Complex I (also known as NADH Coenzyme Q oxidoreductase) is found in the mitochondria of eukaryotes and the plasma membranes of some bacteria. It couples the oxidation of NADH and the reduction of ubiquinone to provide the electrical gradient necessary to produce ATP. Complex I and its partner enzyme, complex III, are the primary sites of reactive oxygen species production in mitochondria. Mutations in complex I are associated with many disease conditions, including Parkinson's disease and Alzheimer's disease.
An antioxidant compound produced by the plant Curcuma longa, a member of the ginger family. Curcumin exhibits a wide array of beneficial health effects, including anti-inflammatory, anti-cancer, and anti-diabetes properties. It is responsible for the bright yellow pigment of turmeric, a type of spice commonly used in Indian food.
A broad category of small proteins (~5-20 kDa) that are important in cell signaling. Cytokines are short-lived proteins that are released by cells to regulate the function of other cells. Sources of cytokines include macrophages, B lymphocytes, mast cells, endothelial cells, fibroblasts, and various stromal cells. Types of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factor.
A neurotransmitter best known for its role in motor, motivation, and pleasure control. Dopamine also functions as a paracrine (cell-to-cell) hormone in other parts of the body. It is derived from tyrosine and is the precursor to norepinephrine and epinephrine. Some evidence suggests that dopamine may also be involved in pain modulation.
A genus of flies, often called "fruit flies," that has been heavily used in research in genetics and is a common model organism in developmental biology. Fruit flies are popular experimental animals because they are easily cultured en masse out of the wild, have a short generation time, and mutants are readily obtainable.
The single layer of cells that lines the interior of the blood and lymphatic vessels. The endothelium participates in blood flow, platelet aggregation, and vascular tone. It also regulates inflammation, immune function, and angiogenesis. Endothelial dysfunction is a systemic pathological condition broadly defined as an imbalance between vasodilating and vasoconstricting substances produced by (or acting on) the endothelium. It is a robust predictor of heart attack and stroke risk.
Any of a group of complex proteins or conjugated proteins that are produced by living cells and act as catalyst in specific biochemical reactions.
Amino acids that cannot be synthesized by the organism, but must be supplied via diet. The nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine.
An antioxidant compound produced by the body’s cells. Glutathione helps prevent damage from oxidative stress caused by the production of reactive oxygen species.
The years of a person’s life spent free of disease.
A family of proteins produced by cells in response to exposure to stressful conditions. Heat shock proteins are expressed in response to heat as well as exposure to cold and UV light, and during wound healing and tissue remodeling. Many heat shock proteins function as chaperones by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by cell stress. A 30-minute 73ºC sauna session in healthy young adults has been shown to cause a robust and sustained increase in the production of heat shock proteins for up to 48 hours afterward.[1]
- ^ Shields, Richard K; Iguchi, Masaki; Littmann, Andrew E.; Chang, Shuo-Hsiu; Wester, Lydia A.; Knipper, Jane S. (2012). Heat Stress And Cardiovascular, Hormonal, And Heat Shock Proteins In Humans Journal Of Athletic Training 47, 2.
Iron overload, also known as hemochromatosis, indicates accumulation of iron in the body from any cause. The most important causes are hereditary haemochromatosis (HHC), a genetic disorder, and transfusional iron overload, which can result from repeated blood transfusions. Affected individuals over the age of 40 or who have high serum ferritin levels are at risk for developing cirrhosis and have a higher risk of hepatocellular carcinoma as well.
An organism’s ability to maintain its internal environment within defined limits that allow it to survive. Homeostasis involves self-regulating processes that return critical bodily systems to a particular “set point” within a narrow range of operation, consistent with the organism’s survival.
Biological responses to low-dose exposures to toxins or other stressors such as exercise, heat, cold, fasting, and xenohormetics. Hormetic responses are generally favorable and elicit a wide array of protective mechanisms. Examples of xenohormetic substances include plant polyphenols – molecules that plants produce in response to stress. Some evidence suggests plant polyphenols may have longevity-conferring effects when consumed in the diet.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
One of the most potent natural activators of the AKT signaling pathway. IGF-1 stimulates cell growth and proliferation, inhibits programmed cell death, mediates the effects of growth hormone, and may contribute to aging and enhancing the growth of cancer after it has been initiated. Similar in molecular structure to insulin, IGF-1 plays a role in growth during childhood and continues later in life to have anabolic, as well as neurotrophic effects. Protein intake increases IGF-1 levels in humans, independent of total caloric consumption.
An essential mineral present in many foods. Iron participates in many physiological functions and is a critical component of hemoglobin. Iron deficiency can cause anemia, fatigue, shortness of breath, and heart arrhythmias.
A compound that can increase the lifespan of an organism such as rapamycin and resveratrol.
The thousands of biochemical processes that run all of the various cellular processes that produce energy. Since energy generation is so fundamental to all other processes, in some cases the word metabolism may refer more broadly to the sum of all chemical reactions in the cell.
Tiny organelles inside cells that produce energy in the presence of oxygen. Mitochondria are referred to as the "powerhouses of the cell" because of their role in the production of ATP (adenosine triphosphate). Mitochondria are continuously undergoing a process of self-renewal known as mitophagy in order to repair damage that occurs during their energy-generating activities.
Proteins that provide favorable conditions for the correct folding of other proteins. They also repair damaged proteins. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional form. Heat-shock proteins (HSPs) are a well-known type of chaperone.
A family of enzymes whose sole known substrates are glucosinolates. Myrosinase is located in specialized cells within the leaves, stems, and flowers of cruciferous plants. When the plant is damaged by insects or eaten by humans, the myrosinase is released and subsequently hydrolyzes nearby glucosinolate compounds to form isothiocyanates (see definition), which demonstrate many beneficial health effects in humans. Microbes in the human gut also produce myrosinase and can convert non-hydrolyzed glucosinolates to isothiocyanates.
A protein typically present in the cytoplasm of mammalian cells. Nrf2 can relocate to the nucleus where it regulates the expression of hundreds of antioxidant and stress response proteins that protect against oxidative damage triggered by injury and inflammation. One of the most well-known naturally-occurring inducers of Nrf2 is sulforaphane, a compound derived from cruciferous vegetables such as broccoli.
Highly reactive molecules that have the ability to oxidize other molecules and cause them to lose electrons. Common oxidants are oxygen, hydrogen peroxide, and superoxide anion.
A result of oxidative metabolism, which causes damage to DNA, lipids, proteins, mitochondria, and the cell. Oxidative stress occurs through the process of oxidative phosphorylation (the generation of energy) in mitochondria. It can also result from the generation of hypochlorite during immune activation.
A neurodegenerative disorder that affects the central nervous system. Parkinson’s disease is caused by destruction of nerve cells in the part of the brain called the substantia nigra. It typically manifests later in life and is characterized by tremors and a shuffling gait.
The observable physical characteristics of an organism. Phenotype traits include height, weight, metabolic profile, and disease state. An individual’s phenotype is determined by both genetic and environmental factors.
An extremely rare genetic disorder in which symptoms resembling aspects of aging are manifested at a very early age. People born with progeria typically live to their mid teens to early twenties. Although the term progeria applies strictly speaking to all diseases characterized by premature aging symptoms, it is often applied specifically in reference to Hutchinson–Gilford progeria syndrome (HGPS).
Protein complexes inside cells that degrade misfolded, damaged or unneeded proteins via proteolysis, which is a chemical reaction that breaks peptide bonds.
Overtime proteins unintentionally accumulate damage from reactive oxygen and nitrogen species. These compromised proteins aggregate together and can promote aging as well as progressive diseases such as Alzheimer's and Parkinson's disease.
The 3-dimensional structure of a protein. The structure of a protein is determined by its amino acid constituents, the interaction of its amino acids with each other, and the interaction of its amino acid constituents with the environment surrounding the protein. The conformation then determines how the protein functions and how long its half-life is.
A portmanteau of the words protein and homeostasis. Proteostasis is maintained through the competing and integrated biological pathways within cells that control the biogenesis, folding, trafficking and degradation of proteins present within and outside the cell. Proteostasis deteriorates with age. As a result, the prevalence of age-related protein misfolding diseases, such as Alzheimer’s disease and Parkinson’s disease, increases.
A study in which people are randomly allocated to receive one of several clinical interventions. One of these interventions is the standard of comparison or control. The control may be a standard practice, a placebo, or no intervention at all.
A compound initially developed as an antifungal agent. This use was abandoned, however, when it was discovered to have potent immunosuppressive and antiproliferative properties due to its ability to inhibit one of the complexes of mTOR (mTORC1). Rapamycin has since shown interesting lifespan extension properties in animals.
Oxygen-containing chemically-reactive molecules generated by oxidative phosphorylation and immune activation. ROS can damage cellular components, including lipids, proteins, mitochondria, and DNA. Examples of ROS include: peroxides, superoxide, hydroxyl radical, and singlet oxygen.
A related byproduct, reactive nitrogen species, is also produced naturally by the immune system. Examples of RNS include nitric oxide, peroxynitrite, and nitrogen dioxide.
The two species are often collectively referred to as ROS/RNS. Preventing and efficiently repairing damage from ROS (oxidative stress) and RNS (nitrosative stress) are among the key challenges our cells face in their fight against diseases of aging, including cancer.
Otherwise known as Brewer’s or Baker’s yeast, is believed to have been initially isolated from the skin of grapes. S. cerevisiae is one of the most intensively studied eukaryotic model organisms in molecular and cell biology. Many proteins important in human biology were first discovered by studying their homologs in yeast, especially proteins involved in cell cycle, signaling, and protein-processing. In the field of aging, this organism has been responsible for discovery of mammalian genes affecting aging more than any other model organism and continues to be used extensively for the study of DNA damage and repair mechanisms.
Senescence is a response to stress in which damaged cells suspend normal growth and metabolism. While senescence is vital for embryonic development, wound healing, and cancer immunity, accumulation of senescent cells causes increases inflammation and participates in the phenotype of aging.
A change in one nucleotide DNA sequence in a gene that may or may not alter the function of the gene. SNPs, commonly called "snips," can affect phenotype such as hair and eye color, but they can also affect a person's disease risk, absorption and metabolism of nutrients, and much more. SNPs differ from mutations in terms of their frequency within a population: SNPs are detectable in >1 percent of the population, while mutations are detectable in <1 percent.
An isothiocyanate compound derived from cruciferous vegetables such as broccoli, cauliflower, and mustard. Sulforaphane is produced when the plant is damaged when attacked by insects or eaten by humans. It activates cytoprotective mechanisms within cells in a hormetic-type response. Sulforaphane has demonstrated beneficial effects against several chronic health conditions, including autism, cancer, cardiovascular disease, diabetes, and others.
Distinctive structures comprised of short, repetitive sequences of DNA located on the ends of chromosomes. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division. When the telomeres on a cell’s chromosomes get too short, the chromosome reaches a “critical length,” and the cell stops dividing (senescence) or dies (apoptosis). Telomeres are replenished by the enzyme telomerase, a reverse transcriptase.
A dye widely used to visualize and quantify the presence of misfolded protein aggregates called amyloids.
A protein that binds to specific DNA sequences, thereby controlling the rate of transcription of genetic information from DNA to messenger RNA. A defining feature of transcription factors is that they contain one or more DNA-binding domains, which attach to specific sequences of DNA adjacent to the genes that they regulate.
A fat-soluble vitamin stored in the liver and fatty tissues. Vitamin D plays key roles in several physiological processes, such as the regulation of blood pressure, calcium homeostasis, immune function, and the regulation of cell growth. In the skin, vitamin D decreases proliferation and enhances differentiation. Vitamin D synthesis begins when 7-dehydrocholesterol, which is found primarily in the skin’s epidermal layer, reacts to ultraviolet light and converts to vitamin D. Subsequent processes convert D to calcitriol, the active form of the vitamin. Vitamin D can be obtained from dietary sources, too, such as salmon, mushrooms, and many fortified foods.
A foreign substance that is introduced into the body from the environment and is subsequently metabolized. Xenobiotics can exert multiple effects (good or bad) on the body by disrupting or interacting with cellular communication pathways that regulate growth, development, and normal physiological function. They are subject to extensive biotransformation in the human body via Phase 1 and Phase 2 metabolism, with a goal toward elimination. Examples of xenobiotics include drugs, pollutants, and plant-based dietary compounds.
An adaptive physiological response in which bioactive compounds, produced by environmentally stressed plants, induce beneficial stress response pathways in animals, including humans. Xenohormetic responses ultimately confer stress resistance and longevity and may explain some of the beneficial effects of plant-based foods. The term xenohormesis stems from two terms: xeno (stranger) and hormesis (a protective physiological response induced by mild stressors). Polyphenols, isothiocyanates, and other plant compounds are thought to exhibit some of their beneficial properties by inducing a type of xenohormesis.
Attend Monthly Q&As with Rhonda
Support our work

The FoundMyFitness Q&A happens monthly for premium members. Attend live or listen in our exclusive member-only podcast The Aliquot.