#40 Dr. Eric Verdin on Ketogenic Diet, Longevity, Beta-Hydroxybutyrate, HDAC Inhibitors, & NAD+
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
Eric M. Verdin, M.D. is the fifth president and chief executive officer of the Buck Institute for Research on Aging and is a professor of Medicine at UCSF. Dr. Verdin's laboratory focuses on the role of epigenetic regulators in the aging process, the role of metabolism and diet in aging and on the chronic diseases of aging, including Alzheimer’s, proteins that play a central role in linking caloric restriction to increased healthspan, and more recently a topic near and dear to many of you, ketogenesis. He's held faculty positions at the University of Brussels, the NIH and the Picower Institute for Medical Research.
A cyclic ketogenic diet and its effects on memory and longevity.
In this podcast, we talk broadly about many interesting areas in the field of aging, but of special interest is his lab’s recent publication on a cyclic ketogenic diet (alternating between keto and a regular diet) that was able to improve longevity and memory in mice when started in mid-life.
"The most remarkable thing we saw is that these older mice on the ketogenic diet showed actually better memory than younger mice, and we did not see the loss of memory function that one would normally see associated with the aging process."- @EricVerdin Click To Tweet
Some of the key topics discussed in this episode include...
- The effects of a low protein cyclic ketogenic diet beginning in midlife (12 months of age) in male mice. The result? Increased healthspan and improved memory. Dr. Verdin explains how the cyclic ketogenic diet decreased insulin, IGF-1, and mTOR signaling and decreased fatty acid synthesis, and increased PPAR-alpha (which promotes beta-oxidation and mitochondrial biogenesis in muscle).
- How this diet shares some qualities with fasting.
- Some of the possible reasons why the cyclic ketogenic diet created such a striking improvement in memory even when compared to younger mice.
- How beta-hydroxybutyrate, which is the major circulating ketone body during fasting and nutritional ketosis, may, in addition to being an energy source, regulate inflammation and gene expression by acting as a signaling molecule by inhibiting what are known as class 1 histone deacetylases (HDACs).
- How this inhibition of class 1 HDACs leads to the increased expression of notorious longevity gene Foxo3, which may help explain why mice given an exogenous beta-hydroxybutyrate ester had lower markers of inflammation and oxidative damage, which are physiological contributors to the aging process.
- The role of the nicotinamide adenine dinucleotide (NAD+) in the aging process and how replacing declining levels (or preventing them from declining in the first place) may prove to be an important anti-aging strategy.
- Some of the reasons why NAD+ might be declining with age, its role in DNA damage repair via an enzyme known as PARP, and what the literature says about the NAD+ precursor nicotinamide riboside.
- How a special class of enzymes called sirtuins, also known to be activated by caloric restriction and caloric restriction mimetic resveratrol, is tightly correlated with the level of NAD+ and how this "energetic currency" rises in response to fasting.
- The role of the sirtuin enzymes in regulating mitochondrial function, neuronal functions, stem cell rejuvenation and why they may be important in delaying the aging process.
Learn more about Dr. Eric Verdin
- @EricVerdin on Twitter
- Eric Verdin, M.D., President and CEO - Buck Profile
- Verdin Lab - Buck Institute for Research on Aging
- List of Publications
- Wikipedia Profile on Eric Verdin, M.D.
Relevant publications
- Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice
- A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice
- Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor.
- Long-term calorie restriction in humans is not associated with indices of delayed immunologic aging: A descriptive study
- NAD+ in aging, metabolism, and neurodegeneration
-
"A child born in the year 2007 has a 50% change of living to be 104 years old."
-
The four categories of aging research: exercise, nutrition, mimetics, and rejuvenation.
-
Dr. Verdin discusses the role that the insulin pathway plays in aging.
-
-
Plausible genetic pathways that might be responsible for the longevity effects related to the ketogenic diet (related to BHB).
-
Beta-hydroxybutyrate was initially underestimated in its role as an HDAC inhibitor due to the required concentration, which is now known to be physiologically normal (especially in the presence of a ketogenic diet or fasting).
-
The big question of whether or not an exogenous ketone esters can recapitulate the spectrum of effects afforded by a ketogenic diet.
-
How high protein compensation during a ketogenic diet may actually negate some of the positive health benefits through overactivation of mTOR & IGF-1.
-
Dr. Verdin explains how the wide genetic variation among humans complicates the application of discoveries from mice to humans and highlights why identifying biomarkers is important for understanding the effect of interventions on specific individuals.
-
There is exciting new technologies that can predict your biological age including artificial intelligence that predicts your age based off facial recognition.
-
Replenishing falling levels of NAD+ associated with aging may be a strategy for reducing some symptoms of aging and neuronal cell death.
-
How PARP, a family of proteins activated by a type of DNA damage known as single strand breaks, may be one cause for the cellular NAD+ depletion associated with aging.
-
-
Thoughts about the safety of the ketogenic diet on liver health.
-
-
Reference to Valter Longo’s research on prolonged fasting. See episode.
- Rhonda: Hello, everyone. Welcome back to another episode of the “FoundMyFitness” podcast. I am sitting here with Dr. Eric Verdin, who is the president of the Buck Institute for Research on Aging. Now, I recently saw a quote from you that said you stated that a child born in the year 2007 had a 50% chance of living to be 104 years old.
- Dr. Verdin: Yes.
- Rhonda: First of all, yes, you did. Can you explain what you mean by that? Because that’s pretty exciting.
- Dr. Verdin: I think it says what it says based on the projection of where the progression and lifespan that has happened during the last 100 years, which has been about 2 years per decade. I think scientists at Berkeley actually have been able to… This data comes out of a book that was based on data from Berkeley suggesting that a child born in 2007 has a 50% chance of living to 104.
- Rhonda: After reading that quote, I was thinking, “Well, maybe some of these lifestyle interventions that we know to regulate the aging process that we know that can have a positive effect on healthspan. Can you maybe tell people a little bit about what are some of the main lifestyle interventions that are known to regulate the aging process at least in animal models?
- Dr. Verdin: Yes. And in humans as well. I think, there are, you know, four broad categories of things that are being considered by the aging field. One is exercise is to this day the surest, best intervention that we have to increase healthspan and lifespan. The second one is nutrition, and there’s a lot of research going on today trying to understand what is it about nutrition and carbohydrates versus fat, versus proteins, what is the relative role of all these nutrients in your lifespan and healthspan. The third one, which is actually an active field of investigation, is the identification of molecules that mimic either exercise or the sort of exercise mimetic or mimic restriction in terms of nutrients, what we call calorie restriction mimetics. And, finally, the last part of the whole aging field is the idea of rejuvenation. So, the first three approaches are geared towards slowing down the process of aging. Rejuvenation takes the approach that once the aging has occurred how can you repair it and how can you fix it. So, I think, you know, we have programs here that are studying all four different approaches. I think you’re probably familiar with Jack LaLanne, who is one of the gurus that started the American sort of infatuation with exercise. He lived to 100 years old, and he said that, “Exercise is king. Nutrition is queen. Put them together, and you have a kingdom,” which I think is really true. You know, for me and many of my colleagues, I think exercise and nutrition is the cornerstone of what we’re trying to do today until we have better drugs.
- Rhonda: It’s a beautiful quote. And in terms of the nutrition, when I think of nutrition, you mentioned, you know, the macronutrient content, trying to understand the ratios of carbohydrates, and protein, and fat, I often think of the micronutrients when I think of nutrition. My mentor for my postdoc was Dr. Bruce Ames, and, of course, you know, he’s very focused on micronutrients, vitamins, minerals, essential fatty acids, and amino acids. But, in the past couple of decades, the research has seemed to show that limiting these certain macronutrients, protein, fat, carbohydrates, you sort of tweak the amount that you take in, you can alter the way an animal ages at least in terms of the way their tissues are aging. You’re not necessarily going to, you know, increase their maximum lifespan, but you may increase their average lifespan, which is…
- Dr. Verdin: Yes, I think, in the last 20 years, we’ve learned a lot. And, a lot of these research has been actually causing a reevaluation of some of the public policies that have been enacted in the last 20 and 30 years in terms of what should you eat, when should you eat. So, a lot of work is ongoing today and actually generating lots of really interesting data. Along with this, the basic science of aging, I think what people have studied is trying to understand what are the pathways that control aging in C. elegans, in Drosophila, the fruit flies, in the little nematode, or in mice. One of the major pathways that has emerged is insulin signaling pathway. That was the work of Cynthia Kenyon back in 1990 showing that the insulin signaling pathway is one of the major pathways that controls aging. Well, if you know this, you can already sort of backtrack and say, “Well, what does insulin do?” It’s the major hormone that allows you to utilize carbohydrates, and so the implication of this is the more carbohydrates you eat the more you activate the insulin signaling pathway, and the prediction would be that the more or the faster you age. And I think the real data really suggests this model. Now, it lies completely in the face of what we’ve assumed to be correct. You can walk into any store and find low-fat diet and low-fat products. Turns out that we really believe that the culprit is more carbohydrates. And a recent paper just came out, which is really remarkable, showing, analyzing in thousands of humans fraction of their total calorie intake that is represented by carbohydrates, and they were able to…just by interviewing them and asking, you know, “What do you eat?” And what they showed in this paper is all-cause mortality was directly correlated to the amount of carbohydrate that one eats. So, the people who ate the least amount of carbohydrates showed the lowest all-cause mortality.
- Rhonda: Wow. Did they differentiate between refined carbohydrates and, for example, vegetables which are carbohydrates?
- Dr. Verdin: They did. They didn’t. And I think, you know, this is obviously not all carbohydrates are created equal, but I think…
- Rhonda: Possible confounder.
- Dr. Verdin: Irrespective, total carbohydrate was a very strong predictor. But, obviously, you know, if your total carbohydrates intake… It’s very hard to eat a high amount of carbohydrates that are all sort of a low absorption type of carbohydrates. So, typically, the people who eat a lot of carbohydrate will eat a lot of the bad ones as well.
- Rhonda: Right. Yeah. Most people that are eating that are probably eating more of a standard sort of American diet, you know, where it’s chips, crackers, and cookies, and I think people that are following more like of a Paleolithic type of diet, where they’re eating, you know, whole foods, and meat, and nuts, probably would eat their carbohydrate intake. The bowl could be from vegetables, things like that. But, you’re mentioning a really important point, is that the carbohydrate intake and the insulin signaling pathway, these things, carbohydrates regulate that, but also you limit your carbohydrate intake when you’re fasting, right? I mean…
- Dr. Verdin: You limit all of your intake.
- Rhonda: Oh, yeah, all of it essentially, yeah. But, your insulin signaling goes down, right? And, maybe you could talk a little bit about, you know, fasting, because fasting is one of the well-known, you know, dietary interventions that can regulate the way we ate.
- Dr. Verdin: Yes. And the key question about fasting is there’s growing evidence that it is beneficial. Intermittent fasting, episodic fasting can actually illicit a response in our bodies and many animal models that are protective against aging. The question is, how does it happen? So, fasting has growing evidence that it actually increases lifespan and healthspan, and the so-called fasting mimicking diets are emerging. A group, Valter Longo’s, suggesting that, you know, these diets have really beneficial effect on healthspan, even in humans. Now, the key question is how do they work, and there are multiple possible mechanism. And it’s possible that the resulting effect is a combination of all of them, of all mechanisms. So, one is decreasing carbohydrate intake. So, that would lead to a decrease in insulin signaling. Second one is restricting protein intake, which would actually lead to decreased mTOR signaling and so on. The third one is induction of ketosis, which is a small nutrient which is generated by your liver during the fasting process, and all works indicates that just ketosis and a ketogenic diet might have beneficial effect all by itself.
- Rhonda: Yeah. So, you recently published a paper. Your lab recently published a paper where you had taken male mice and given them a cyclic ketogenic diet. So, that started in mid-life?
- Dr. Verdin: Actually, it started at one year old.
- Rhonda: One year old, okay.
- Dr. Verdin: So, this is about a third of their normal lifespan. So, that would be equivalent of a 30-year-old human.
- Rhonda: Okay. So, exactly you found that it increased the healthspan of these animals?
- Dr. Verdin: Yes.
- Rhonda: And also there is some effect on the brain?
- Dr. Verdin: Yes. Very interesting data suggesting… Again, the whole experiment started on the idea, the observation that there are many similarities between what happens during ketosis on a ketogenic diet and what happens on calorie-restriction or fasting. And so, with a colleague in the lab, John Newman, a number of years ago, we started asking the question, you know, “Would mice on a ketogenic diet live longer?” So, we spend about a year and a half with John trying to identify the conditions where this mice would be on fats and protein diets, essentially had zero carbohydrates, after one year of age. The problem we had initially is that they actually loved the stuff so they would just devour this diet, and they got fat. So, we were worried that by becoming obese we would sort of counterbalance beneficial effects of the ketogenic diet. So, we spend a bit of time trying to solve this. Eventually, were able to put them one week on, one week off, and that resulted in a stable weight in the mice, and that allowed us to look at their healthspan and lifespan at the end of their lives. And what we’ve saw was an increased medium lifespan by about 10%, so their early mortality was decreased. But, eventually, they ended up having no increase in maximum lifespan. We also found a number of other variables associated with healthspan that were significantly increased, in particular memory. These mice, the most remarkable thing we saw is that these older mice on the ketogenic diet showed actually better memory than younger mice, and certainly we did not see the loss of memory function that one would normally see associated with the aging process. So, I think it was a pretty profound observation. I should say also Jon Ramsey and his colleague at UC Davis conducted the same type of experiment except that instead of putting the mice on a cyclic one week, one week off diet, they allotted fixed portions to the mice so they couldn’t overeat. They gave them the same amount of calories that they would’ve been eating had they been on a normal diet, and so these mice did not get fat and were continuously on the ketogenic diet. And what they saw is essentially parallel to what we saw, but the effects were even a little better, which suggests that the cycling might have been actually a bit stressful for the mice over the long run. And so I think we’re very excited by these two studies, because they were conducted independently. We found out only that we were pursuing the same thing right at the end at the time of publishing, and then we decided to coordinate publication of the stories, and they came out in the same journal together. So, I think they both reinforced each other. There is really clearly intriguing biology there happening, and this was the first time that this had been reported.
- Rhonda: Wow, absolutely. It’s very exciting. I read your paper. I did not read the parallel paper. I did see the headline for it. But, just to kind of go back to the memory thing that your lab identified, so the ketone body, the major circulating one that’s generated is beta-hydroxybutyrate, and that’s involved in energy metabolism, but also you have found it has other roles in addition to its role in energy metabolism, which we can talk about in a minute. But, what are your thoughts as to why, you know, the memory enhancement was so good or, you know, so robust? Do you think that has to do with energy metabolism, mitochondrial function, or do you know?
- Dr. Verdin: We do not know, but it’s the central question of everything we do right now with respect to the ketogenic diet, is really trying to understand. So, beta-hydroxybutyrate is generated from our own fat during the fasting process. So, when we start fasting, the body needs energy, and we’ll grab it out of our fat cells. These fat cells release fat in the circulation. It goes into the liver, and the liver transforms the fat into beta-hydroxybutyrate. Now, why does it do this? Because our brain cannot use fat as a source of energy. Our brain can only rely on glucose or on beta-hydroxybutyrate. And so the way the system is geared up is the fat gets released into the liver, and the liver distributes the beta-hydroxybutyrate to the whole brain who can then use it and spare glucose. So, why do you want to spare glucose? The reason is that once you are fasting, at least for four hours, the only way you can make glucose is from proteins. And so if you’re in a prolonged fast, you start digesting your muscles to make proteins, to make glucose, and you don’t wanna lose all of your proteins and all of your muscles. So, the glucose-sparing effect of beta-hydroxybutyrate is to spare muscle mass if you’re fasting. So, the system is really carefully engineered to really generate a whole new way for your body to utilize energy when you’re fasting.
- Rhonda: Right. Are you aware of the glucose-sparing effect in the brain where… I know it’s been shown at least in terms of lactate. So the astrocytes in your brain do make lactate, because they’re glycolytic, and then neurons will take it up much like beta-hydroxybutyrate. They go through the same transporter.
- Dr. Verdin: Yes, yes.
- Rhonda: But, when there’s an abundance of lactate, and I don’t know if studies have been done on beta-hydroxybutyrate in the brain looking at this specific thing or not, but the glucose sparing that occurs is the glucose gets shunted into the pentose phosphate pathway to make NADPH, which is a precursor for glutathione synthesis. And so the idea is that under, like, a traumatic situation, like traumatic brain injury, when there’s a lot of oxidative stress happening, you need glutathione to counter from that. So, it’d be interesting to see in your study if you can look at some of those pathways to see if they’re upregulating or making glutathione, because brain aging kind of is like a traumatic insult, but, like, slowly chronic, right? I mean, in a way.
- Dr. Verdin: Well, you know, we published a paper. What got us into this field was an observation that we published in “Science” in 2010 showing that beta-hydroxybutyrate, in addition to being a nutrient, as we just discussed, is also a signaling molecule. And what we found at that time was that beta-hydroxybutyrate, which is quite similar to butyrate, butyrate is a byproduct of bacterial fermentation in our gut. Actually, when we eat fibers, these bacteria will digest the fibers into butyrates, and this butyrate actually circulates in many of us as we live. Butyrate was the first known or first identified inhibitor of HDACs, histone deacetylases, which are epigenetic regulators, and so that suggested that maybe beta-hydroxybutyrate might be an endogenous regulator of these HDACs. Now, the reason why we were interested in HDACs is because they have been linked to aging as well. Stephen Helfand’s work has shown that an enzyme called Rpd3 in yeast is actually a regulator of the sirtuins, which are themselves a regulator of aging. So, there’s a pathway that’s in yeast that’s been established actually in Drosophila as well linking Rpd3 to sirtuin to aging, the aging process, and these HDACs that I’m talking about are in the same pathway. So, that got us to start thinking, “Could beta-hydroxybutyrate be also an HDAC inhibitor?” and it was. And then this implicated that it might actually be able to regulate gene expression, and some of the key targets that we found were enzymes such as FOXO3, which is a major sort of transcription factor in humans linked to aging, and it turns out that FOXO3 actually controls the response to oxidative stress. So, there’s another link there that, you know, brings not only the pentose phosphate, NADPH, but beta-hydroxybutyrate actually protects against oxidative stress, both as a nutrient, but also as a transcription or regulator.
- Rhonda: That’s super cool. Do you know what levels of beta-hydroxybutyrate are required to sort of flip that switch and…
- Dr. Verdin: It’s a very good question. It’s probably one of the reasons why no one did the experiment before us. Butyrates and beta-hydroxybutyrate are poor inhibitors. They have very low efficiency as HDAC inhibitors. It’s in a millimolar concentration. And, as an inhibitor, nobody wants to work on millimolar inhibitors, because it’s not potent enough. What most ipeople did not realize is that the concentration of beta-hydroxybutyrate in your plasma or in your brain during fasting can go in the millimolar range very quickly. I was reading a paper, discussing these concentrations, and was astounded. I thought, “Well, millimolar concentration, this means that it might really work as an HDAC inhibitor.” And so we tested this by putting a pump under the skin of a mice with beta-hydroxybutyrate and then measuring histone acetylation throughout the mouse, and we found that histone acetylation marks were going up, suggesting that there was indeed an inhibition of HDAC. And I think that was the turning point for us.
- Rhonda: By giving them exogenous beta-hydroxybutyrate.
- Dr. Verdin: Yes, not even the fasting. Because fasting or a ketogenic diet is much more complicated than just giving beta-hydroxybutyrate.
- Rhonda: They were on a normal chow diet.
- Dr. Verdin: Normal chow diet. They were not fasted. And we found that their ketone body levels were going very high in the millimolar range. And, within a few hours, their histones were becoming hyperacetylated. And so that for us, I think, signified that here’s a molecule that is produced during fasting, and they’re conditions that we know are good for your health. So, ketogenic diet has been indicated, has been shown to have some beneficial effect under a series of circumstances. I was on this. We had a potential signaling mechanism, and that’s what we’ve pursued.
- Rhonda: Yeah. I do remember saying that paper was published in “Science” at 2010 or something like that.
- Dr. Verdin: Or ‘11.
- Rhonda: Yeah. I do remember that paper. Very super cool paper. I actually talk about it quite often, you know, when I’m talking about beta-hydroxybutyrate. So, what do you think of exogenous ketone esters? Like, do you think that they’re safe? Is that something that you know about or have thought about?
- Dr. Verdin: Well, we’ve thought about it. We are experimenting with them as well. It is one of the central questions. It’s most of the beneficial effect that we have seen have been with the ketogenic diet. Again, that’s very different from a high beta-hydroxybutyrate level. And one of the remaining or at least the next step in this whole field is really to understand what can we recapitulate with beta-hydroxybutyrate alone, and I think we’re working actively on this and so are many other groups. The ketogenic diet itself it’s important to realize is not an easy diet to live on. You know, I don’t mean to be diminishing the merit… Actually, I highlight the merit of people who are on a long-term ketogenic diet. It takes a lot of discipline. You basically cannot eat a significant amount of carbohydrates. Many people who have been on this diet for a long time actually tout its benefits and the fact that they feel very healthy and they feel very present. There are a lot of brain effects linked to ketogenic diet. Now, big question is what can we replicate just by administering BHB, beta-hydroxybutyrate, alone.
- Rhonda: Yeah. I think that you’re absolutely right, you know, and not to mention that with a ketogenic diet there’s also… You know, some people can’t really do them. You know, there are certain gene polymorphisms. I know there’s one in the PPAR-alpha gene which is important for the whole process of making ketone bodies and that people have that they actually can’t do that very well, and so it can be sort of dangerous, and they can have inflammation. Sort of the opposite profile will happen, you know, so it’s certainly something to consider. And then not to mention, like, you found in your paper that you just published this year…was it last month?
- Dr. Verdin: Yeah.
- Rhonda: Yeah, last month. That you found, you know, there were multiple changes going on. I think there was a decrease in insulin, obviously, and IGF-1, and mTOR activity went down. And then, you know, so that’s something you’re not gonna just get from beta-hydroxybutyrate. Maybe, we don’t know. I don’t…
- Dr. Verdin: We don’t know. I suspect, not for the mTOR part, but we compared the ketogenic diet to a high-fat diet which was not ketogenic, which mice ate a large amount of fat and enough carbohydrate to suppress ketogenesis. And one of the biggest difference, I think, actually between these two was the activation of PPAR-alpha. And this is also very exciting, because that indicates that maybe a lot of the beneficial effect that we see on the ketogenic diet come from PPAR-alpha activation. We’ve been reading that literature. There’s quite a bit of information there that really hasn’t been pursued as a next direction to try to dissect these effects.
- Rhonda: Because PPAR-alpha’s doing something to the mitochondria. What’s the main…
- Dr. Verdin: It’s a key enzyme of the fasting response, but it seems to be more highly activated in response to the ketogenic diet than to a pure high-fat diet.
- Rhonda: What about fasting, if you compare fasting to…
- Dr. Verdin: It is activated. We did not compare to fasting. We compared it just high-fat to…but I think it just points to a new direction in which we can start dissecting what is the role of PPAR-alpha in these responses.
- Rhonda: But, with your diet, you limited the protein intake, and you didn’t limit the caloric intake, that was the other study?
- Dr. Verdin: No. The protein intake was actually 10% isocaloric between all of the diets that we tested, and the other group did the same thing. So, we were very careful not to change the protein intake. And the other group actually did change a bit the protein content, which could be taken as a confounding variable. So, I think we were very careful in not changing the protein count there.
- Rhonda: Is that standard for… You know, ketogenic diets have grown in popularity. And, you know, so do you think that the ketogenic diet you used you hadn’t have done a 10% protein if they had done a little bit more? I’m not sure if maybe that technically wouldn’t have even been a ketogenic diet. But would it have changed the IGF-1 mTOR axis as much, or…
- Dr. Verdin: You’re bringing an important point, which is that a number of people who go on a ketogenic diet tend to compensate by increasing their protein intake, which might actually put them at risk for exactly what you’re describing, increase IGF-1 signaling and increase actually risk of cancer. There’s a really close correlation between your IGF-1 level and your risk of cancer. So, I think this is something that needs to be considered in the future for people who are on a long-term ketogenic diet.
- Rhonda: And with the caloric restriction diets as well, there was a study I remember reading that humans that end up doing caloric restriction, like just the whole society of caloric restriction, they eat, you know, 30% less calories than they normally would or whatever, something like that. But, they end up eating a higher percentage of protein, because it’s more satiating. So, humans sort of naturally gravitate to eating more protein when they’re eating less food. And so what’s interesting is a lot of those… I think you even published a study recently looking at biomarkers of aging in lymphocytes or monocytes?
- Dr. Verdin: Yes. And we saw no difference between people in calorie restriction. This is something important to consider when lot of the work that we do in the lab are done on mice that are isogenic or congenic, so these are mice that are also mated to one another. When you bring discoveries to the human population, it is critical to take into consideration the incredible variation between different people and how they might respond to the same interventions. We know from everything that we’ve studied in the medicine that I am not the same as you are and you are different from your neighbor in many biological responses, and that includes response through calorie restriction as suspected. In a number of people, it would actually do them harm, and in a number of people, it will do them good. We know this when we test it from strains of mice by calorie restriction. Half of the strains actually responded by shortening of lifespan, the other half by an increase in lifespan. So, we take calorie restriction, for example, as a universal modification that will increase lifespan. It’s not what is seen in the literature, and I would say the same is going to be even more true for humans. So, I think this brings out one of the key things that is lacking in the field of aging, is the identification of biomarkers that would allow us to test on an individual basis whether the intervention or the modifications that you’re imposing is actually pointing you in the right direction or in the wrong direction. And I would caution anyone who’s considering doing something long-term in terms of their health to be very careful in how they feel and how…because we don’t have these biomarkers of aging. They’re emerging, but they’re not validated, and certainly not in the human populations. They’re emerging in mice models and other models, but sort of that’s really where we will be going in the next few years, is really having true biomarkers that allow us to predict whether a given intervention is beneficial or actually hurtful.
- Rhonda: Yeah. What do you think are the top three right now we have for biomarkers for aging?
- Dr. Verdin: I can tell you the one that are really exciting me. Alexander Zhavoronkov has a company called Insilico Medicine that is building biomarkers of aging based on facial recognition and based on metabolites. Just consider how…
- Rhonda: Metabolites.
- Dr. Verdin: Yeah. So, consider how you and I can look at a human and pretty much guess what their age is for most people, and consider the fact that where this is coming from is the fact that we’ve lived, and we’ve met thousands of people, and we’ve heard about their ages, and we sort of build a database of information. So, what Alex and his company have done is they’ve taken the pictures of thousands of people and fed them to a deep neural network and artificial intelligence, and the computer has been able to learn how to recognize and to link on average what is the face of a 7-year-old versus a 60-year-old. So, you can do this, and they will tell you what the computer thinks your age is, and for some people you fall right on, but some number of people you find that your age based on your face actually looks younger or older. So, this would be a reflection your biological age. He’s also been able to do this with the blood markers. With 20, or 30, or 40 sort of usual blood markers, by screening a large number of people, he can actually generate curves, and then you can put in your blood values and see where you fit. So, that’s one approach. BioAge is a company in the Bay Area that is actually pursuing the same idea, looking at artificial intelligence and biomarkers in plasma or in urine and so on for identifying your age. And, finally, one big area is the whole idea of the epigenetic clock.
- Rhonda: Steve Horvath, yeah.
- Dr. Verdin: Steve Horvath and Trey Ideker have shown that there are changes in DNA methylation that are actually pretty closely chronological age. So, I think all of those together are emerging as indicative that there are markers that we can reliably measure. The key is how many of those do we need, how can we reduce this to something that can be a very strong predictor, not so much of your chronological age, but of your biological age.
- Rhonda: Right. Yeah. I think there was a study I did read, maybe it was the company that you mentioned, where there was a panel of blood biomarkers that they looked at, like, telomere length, and immunosenescence, and, you know, the standard panel. And then they had people, I think it was people, that were asked to identify their facial age or something like that, and then they asked their chronological age, and their facial age actually matched their biological age more than their chronological or something like that.
- Dr. Verdin: Exactly. We all know in our acquaintances some people that actually look younger and some people that look older. And we know this because we have a deep neural network in our head that has allowed us to build, you know, reference points so that we can pretty much guess how old people are. And the computer does it even more efficiently, because it can be fed tens or hundreds of thousands of pictures and…
- Rhonda: That’s cool. I want to try it out.
- Dr. Verdin: Yeah. It is actually pretty cool.
- Rhonda: I’m gonna be getting my telomeres tested, you know. Just I don’t know how reliable that’s gonna be. We’ll see about it. You know, I’ve been getting less sleep than usual, because I’m a new mother, and that’s what I’m trying to effect, but it’ll be interesting to see. Anyways, I sort of digressed. I kind of wanted to ask you a little bit about, you know, we’re talking about fasting, and we talked a lot about, you know, insulin signaling decreasing, IGF-1. We’ve talked about before in the podcast before with Valter Longo and others just how protein restriction seems to really regulate that, and that also regulates aging process, mTOR as well. But, something else that really seems to change with fasting is the NAD levels, and that’s something that, you know, your lab has studied extensively with the sirtuins. So, maybe could you talk a little bit about… Because NAD is also extremely exciting to me, and it’s pretty popular these days as well.
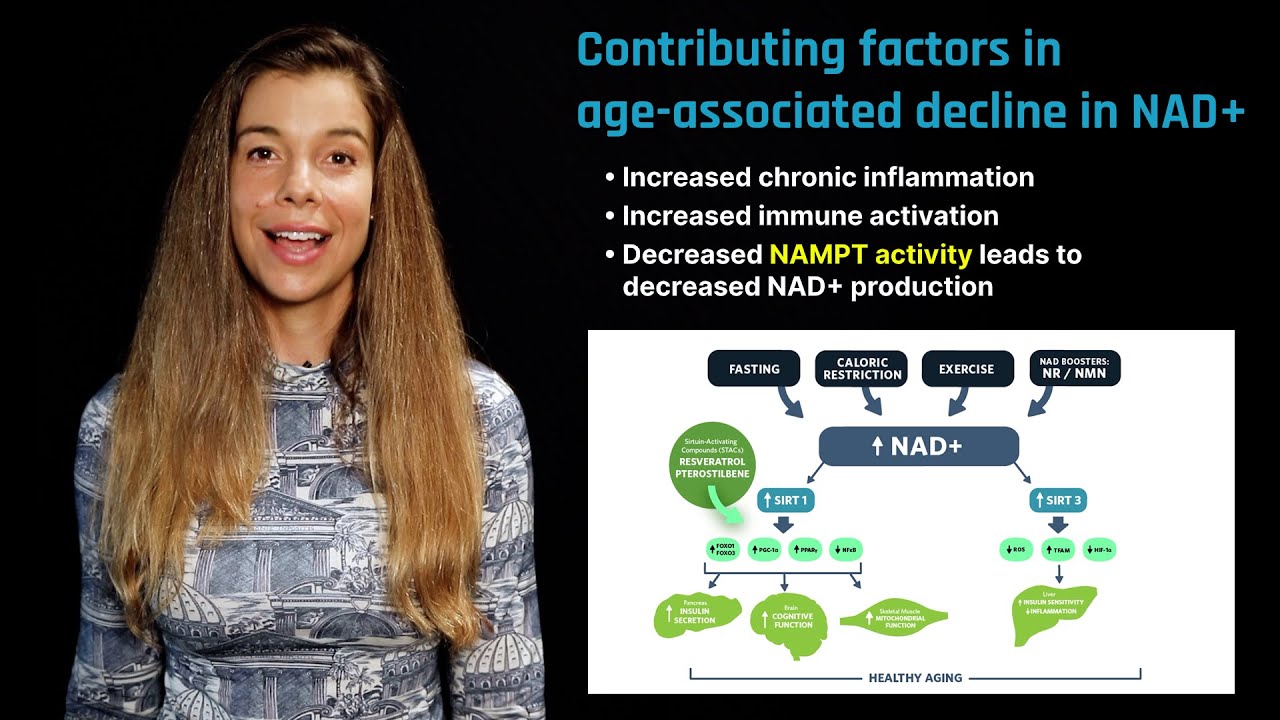
- Dr. Verdin: Yes. So, NAD has emerged as one of these critical intermediary metabolites. Think of ketone bodies, NAD, all, I call them currencies. I mean, so think about the organism as a country. You need to circulate energy, and NAD is one way that our body is utilizing within the cell to convert and transfer energy. It’s almost like the Brinkman truck. It carries the money. And NAD is a hydride acceptor. While we oxidize foods, it can actually serve as an acceptor for electron, and then it can transfer them, for example, to the respiratory chain. So, it’s one way for the energy to be circulated within the cell, and there’s growing evidence that its level decrease during aging. Why that happens is still one of the big mysteries, and so this has yielded a whole approach. They’re trying to understand, first, what are the consequences of decreased NAD levels, and one of the consequences is that enzymes like sirtuins which rely on NAD to exert all of their beneficial activities actually function less well. That’s what happens during aging. But, also many other enzymes that are involved in our metabolism are relying on NAD, and so they function less well, so your intermediary metabolism functions less well. The sirtuins, which are global regulators, function less well. Your… Anyways, I lost my…
- Rhonda: You’re basically falling apart.
- Dr. Verdin: Yeah. Essentially, you know, everything becomes a little less efficient. So, out of these discoveries came the idea that maybe we should replenish the decreasing levels of NAD, and so this has yielded some discoveries, such as nicotinamide riboside, nicotinamide mononucleotide, which are now being taken by a lot of people with the hope that they will, you know, correct some of these problems. One word of caution I think there is, we do not know why these levels decrease. They could decrease because we have decreased production of NAD, but it could also decrease because we have accelerated destruction of NAD, which means, if it’s accelerated destruction, bringing more into it is sort of like pouring more NAD in a leaky sink. So, I think a lot of our work right now is trying to understand what is the cause of the decrease in NAD during aging, because I think it will yield very different solutions. If you find that there’s a leaky sink, we’ll work at plugging the sink versus keeping pouring water.
- Rhonda: I have a theory.
- Dr. Verdin: Yes.
- Rhonda: So, I know, you know, I did a lot of work with DNA damage, and knowing that one of the main enzymes…
- Dr. Verdin: PARP.
- Rhonda: Exactly. I mean, if you think about… So one of the main enzymes that repairs damage as we age, DNA damage, PARP, requires NAD, and it’s like if you’re accumulating more and more damage as you age, you have to repair more of that damage, and the more and more damage you’re having, maybe it’s sucking the NAD sort of like almost a triage where you got to keep repairing that damage, so then other things like the mitochondria suffer. So…
- Dr. Verdin: I completely agree, and so there are two major theories right now that have been proposed in terms of why does NAD go down. One is activated PARP, and, indeed, as we age, we accumulate DNA damage. That’s been shown, especially in the brain recently, and so the idea is, by activating PARP, you constantly deplete your NAD levels. The second one is we all have in our body a so-called salvage pathway for NAD, because NAD turns over. There’s this so-called salvaged pathway that allows it to be recycled back to…so we can get NAD from the food, but also we salvage the one that we utilize, and the salvage pathway has been shown to becoming paralyzed while you age. There’s an enzyme called NAMPT that has received a lot of attention. That enzyme tends to be inhibited by chronic inflammation and a high-fat diet. So, it could be a combination of both of these things, but it could be actually working on another mechanism, which is that there might be accelerated destruction by other enzymes beyond par. And getting some exciting results in this direction.
- Rhonda: Cool. So, for people that are not familiar with, like, in terms of what role it plays, you know, in the aging process, you know, it seems as though, at least in some of the studies that I’ve seen, that mitochondrial function really seems to be important and that you’ve shown that the sirtuin-3 in the mitochondria itself seems to be really important for the mitochondrial function, keeping your mitochondria young. Mitochondria play a very important role in aging as well.
- Dr. Verdin: A critical role. You know, many of the aging pathways that we know, be it the unfolded protein response, or mitochondrial biogenesis, all point to efficient mitochondria as one of the key ways to stay young. And one reason is because this is a way so you can generate energy from glycolysis, which is glucose, carbohydrates, which, as we know, is linked to aging, but also via mitochondria via a process called oxidative phosphorylation, and that process is not necessarily depending on glucose, but is depending on an efficient mitochondrial function. So, we talked about NAD-dependent enzyme. Many of these enzymes reside in the mitochondria. And we found that, for example, the NAD supplementation that is being tested in a variety of aging model requires sirtuin-3 quite often, this mitochondrial sirtuin, yes.
- Rhonda: Oh, really? Nicotinamide riboside?
- Dr. Verdin: Yes, for example, the paper we’ve published was focused on the age-associated or noise-induced loss of hearing. So, if you actually subject mice or humans to very acute noise, they have a dose-dependent loss of hearing. You can protect the mice completely from this effect by supplementing with NAD.
- Rhonda: Wow.
- Dr. Verdin: But with nicotinamide riboside, so if you’re going to a rock concert…
- Rhonda: Right, musicians.
- Dr. Verdin: …and you wanna protect yourself, this is definitely something that…
- Rhonda: Harley-Davidson riders.
- Dr. Verdin: Yes. In mice, it actually had an enormous effect, and we found that this effect was dependent on sirtuin-3, so in sirtuin-3 knockout mice, the effect was lost.
- Rhonda: Do you know if that was dependent on sirtuin-3 in the mitochondria of stem cells, or was it just any cell, or do not know?
- Dr. Verdin: So, in the case of hearing loss, it’s dependent on some really uniquely sensitive cells in the inner ear. But, generally, there’s been sort of an assumption in the field that most of the effect of nicotinamide ribosides, protective effect, are dependent on sirtuin-1. I think in…
- Rhonda: Oh, really?
- Dr. Verdin: Yes. And, in some case, it is. In this particular case, noise-induced hearing loss, it was really clearly sirtuin-3.
- Rhonda: So, even the studies that have looked at, for example, I think there was a mouse, had some sort of mitochondrial disorder, even that one was dependent on sirtuin-1?
- Dr. Verdin: No, actually it did not. In that case, I think the assumption was that it might be helping global mitochondrial function. And so there’s a growing number of cases and, for example, DNA-damage-associated disease where you see accelerated aging where people have been testing the effects of supplementation, because those conditions are associated with decreased PARP activity, just like you mentioned. And, in those cases, I think it hasn’t always been clearly mapped what is the real target of NAD that is dependent for the beneficial effect.
- Rhonda: So, NAD levels do decrease with age. Has it been looked at, like, for example, in animals, like in rodents, when they’re fasted, because fasting increases NAD when they’re fasting and they’re older? Does that help rejuvenate the NAD irrespective of their age? Does it…
- Dr. Verdin: I don’t know. I don’t know the answer to that question. It’s a great question. I think, you know, one thing that I remember also about fasting and other critical effect of fasting that is really essential in our thinking is autophagy, and so, you know, there’s growing evidence also that autophagy by itself responds to nutrient availability, so NAD levels, acetyl-CoA levels. This is a new area that people are starting to work in, including my labs, trying to understand what is it about acetyl-CoA, which is another, think about, currency exchanger in the cells, intermediary metabolite that appears to have not only a role as a nutrient, but also as a signaling molecule. Acetyl-CoA is in the Krebs cycle. It’s one of the key intermediary product of the Krebs cycle, but it’s also the substrate for a whole family of enzymes called the acetyltransferases, which are the opposite of what we were talking about, the HDACs, earlier. So, I think there’s really a lot of crosstalk between all of these pathways, and we’re working very actively now on modulation of signaling the acetyl-CoA and these acetyltransferases. Another approach is to mimic calorie restriction in the fasting stage.
- Rhonda: Yeah. I had Dr. Guido Kroemer on the podcast, and he has been studying that as well. You know, it brings up a question I remember I wanted to ask you that was sort of, you know, biology is never just black and white, because I remember him, with his work, he was talking about how important decreasing protein acetylation was for activating autophagy, which happens during fasting, but also, during fasting, you have these beta-hydroxybutyrate, which is now…was it class 2 inhibitor?
- Dr. Verdin: Class 1.
- Rhonda: Class 1 inhibitor of the histone deacetylase, which is kind of the opposite, a little…
- Dr. Verdin: No, because you have to think, so beta-hydroxybutyrate is one step above the sirtuins, and actually the class 1 inhibitors are inhibitors of…
- Rhonda: Oh, they’re inhibitors. That makes sense.
- Dr. Verdin: Exactly. And so it fits perfectly if you go back to the…
- Rhonda: So class 1 is an inhibitor of histone deacetylase. That makes sense now. Okay.
- Dr. Verdin: Yes.
- Rhonda: Thank you. There was a disconnect in my brain when I find to…
- Dr. Verdin: So, the whole idea of… If you think about acetyl-CoA, and we really draw a graph of acetyl-CoA regulating HATs, histone acetyltransferase, and NAD+ regulating the sirtuins, and these enzymes quite often stay in opposition, not all of them. But one of the key players in terms of histone acetyltransferase is p300, and that’s the one we’ve worked on and Guido Kroemer as well has worked on, and it makes sense. You get the same beneficial effects by activating your sirtuins, which lowers acetylation, as you do by inhibiting an acetyltransferase, which also lowers acetylation. So, the message is that nutrient feeding or, you know, low histone acetyltransferase activity or high sirtuin activity all lead to low protein acetylation, which is beneficial.
- Rhonda: And doing the ketogenic diet?
- Dr. Verdin: Regulates one step above.
- Rhonda: Does also, yeah.
- Dr. Verdin: But, obviously, there’s some complexity, because there are some histone marks that are depending on one enzyme. So, this is an oversimplified model, but so far it holds.
- Rhonda: What about autophagy with the ketogenic diet? Is that something… I mean, I don’t know if it would be…
- Dr. Verdin: It would be activated, because clearly it’s a fasting mimicking diet, but we haven’t really studied it directly.
- Rhonda: Yeah. That would be interesting to look at. Also, the other question I would have is, you know, there’s a lot of stress on the liver when you’re doing that sort of diet, right? You’re relying on it for gluconeogenesis to make glucose. You’re oxidizing fat. Has anyone looked at sort of long-term ketogenic diets?
- Dr. Verdin: Yeah. We did. The liver seemed perfectly fine. Just imagine the liver is really…think about it as sort of an energy redistributor. If it’s not dealing with fat during fasting and transforming fat into ketone bodies, it is dealing with food coming in from the intestine during feeding. So, it’s always busy in one way or the other. So, I don’t think that there’s anything to be worried about it generating ketosis. I haven’t heard of any side effects linked to, you know, liver function and so on.
- Rhonda: The other thing would be at least with mice. In your study, you did cyclic. In the parallel study, they did, I guess, continuous, but they restricted the calories, but, you know, mice sort of, and this is something that goes back to something that Guido was talking about in the podcast, they have a notoriously fast metabolism. In fact, he said that, if you fast a mouse for 48 hours, they can lose, like, up to 20% of their body weight, which is, like, phenomenal. I mean, if human could do that, there’d be no obesity, you know. So, do you think that the fast metabolism, you know, coupled with something like the ketogenic diet or even just a lot of the fasting studies in general, is something that can… Like, if we see something like an increase in healthspan, and we see all these positive benefits in rodents, is that something that we may have hope in translating to humans, you think?
- Dr. Verdin: I do think, I mean, it’s important to realize that mice are not humans and that there’s a tall order in terms of proving something that has happened in mice all the way to humans, but that’s our mission. I mean, that’s what we need to do.
- Rhonda: And you have mechanisms.
- Dr. Verdin: And we have mechanisms. That’s the first step. It’s not always predictive, and a large number of drugs that have been shown to be efficient in mice have failed into humans, but that’s the first step that we can take. From our work, I think the next logical step is to go in humans and test.
- Rhonda: Is a ketogenic diet something that you’ve considered trying or doing?
- Dr. Verdin: I have tried. I have tried, and I’ve been on it for about a year. It’s hard to stay on. I called it a somewhat antisocial diet, because you can’t really drink alcohol, you can’t eat a lot of the things that we base, you know, our social interactions on. No breads, no pasta, and very little fruits. I think it’s…
- Rhonda: What about vegetables?
- Dr. Verdin: You can eat some vegetables, but, you know, depending which ones. On a global population basis, it’s not a realistic goal to expect that everyone is going to be on this ketogenic diet.
- Rhonda: Are you gonna continue doing it or…
- Dr. Verdin: No, I’m not on it right now. And I think I find that intermittent fasting is a much easier way to…
- Rhonda: Is that something you practice?
- Dr. Verdin: Yes, yes. Not right now, but I have intermittently.
- Rhonda: So, intermittent fasting, like 12 hours, 16 hours, or 24 hours?
- Dr. Verdin: Well, it’s still a growing question. There’s really interesting work coming out of the Salk Institute on something called time-restricted eating, time-restricted feeding, showing that it’s not just how much you eat or whether you’re eating carbohydrates, but it’s also how often you eat and allowing your everyday for a fasting period. I think that’s probably some of the most important work that we’ve seen recently in this whole field. And, you know, just think about what dietary authorities today are recommending, is three meals and three snacks. I think, based on what we are doing and learning, this is the worst possible way that you can possibly eat. So, I think allowing for each day to have this really restriction in terms of calorie intake allows you to activate all these pathways to suppress in certain secretion, to suppress store, to activate autophagy. All of these, I think, are really critical.
- Rhonda: Yeah. In complete agreement with you. I’ve talked to Dr. Satchin Panda a couple of times on the podcast, a phenomenal researcher, and I’ve been doing time-restricted eating ever since I first spoke with him. Of course, when I was pregnant, I sort of couldn’t of do it as well, but I’m back doing it now, and, you know, I do feel much better when I do it. You know, I try to eat all of my food within a 10-hour window, and I find that that’s the best…
- Dr. Verdin: Fourteen hours is…
- Rhonda: Fourteen hours fasting. You know, I think maybe you can answer this question for me about, you know, it takes anywhere between, like, 12 to 36 hours to deplete your liver glycogen or something like that.
- Dr. Verdin: Actually, it could be faster. Well, instead, from four to six hours, you actually deplete most of it, yes.
- Rhonda: Did it depend on your physical activity levels or things like that?
- Dr. Verdin: Yeah, obviously. Physical exercise will deplete it much more quickly. In terms of entering ketosis, if you were to do it 14 hours, it’s not enough to really gain significant ketosis. You start seeing this at about 16 hours where your level will slowly rise.
- Rhonda: Okay. Yeah. So, doing a 14-hour fast every night is something that I at least try to practice.
- Dr. Verdin: Yes, no nightcap.
- Rhonda: I think that’s the easiest. Yeah, no nightcap.
- Dr. Verdin: No nightcap, no, you know, glass of milk with a spoon of sugar going to bed.
- Rhonda: No wine. But, you get used to it.
- Dr. Verdin: Yes, absolutely.
- Rhonda: You know, you really do. And, you know, like I said, I think it’s the easiest. I haven’t really done a prolonged fast yet. I’ve spoken with Dr. Valter Longo, and, you know, he was talking about the prolonged fast in humans. Like, in mice, I think it’s like 48 hours, but in humans it’s a little like 4 or 5 days. Yeah, and so he has got this fasting-mimicking diet which sort of mimics some of the effects of fasting. I know several people that have done water fast. I haven’t braved it yet. Have you tried doing a prolonged fast?
- Dr. Verdin: Well, I’ve done, you know, Dr. Longo’s diet, the prolon diet.
- Rhonda: Okay. How was that?
- Dr. Verdin: I think it’s a really interesting science. What I like about it is the fact that it really takes this fasting to a scientific level. They’re doing the work. Going on this five-day diet is actually a really interesting experience, because it’s not hard. Maybe on day three it might be a little hard. But one key question that has not been addressed is, if you induce this protective response, how long does it last? And so how often should you do this? Is it something you should do, you know, every three months, or every once a month, or actually once every six months. I think this is the type of information that will allow many of us to really, you know, do the intervention when it’s needed, and I think it’s going to be a lot of research in this whole area that I’m very excited about.
- Rhonda: Yeah. It probably depends also on do you do time-restricted eating every day, and so what you're sort of baseline is, are you, like, obese, or overweight, or metabolically healthy. But, I agree with you. I think it’s an extremely exciting field, and being here at the Buck Institute, you know, for Research on Aging, you’re sort of at the forefront of it all. And then, like you said, there are so many different aspects of the aging biology that’s being researched here. If people wanna learn more about your work and the Buck…
- Dr. Verdin: Yes, so we were the first research institute devoted to aging. We were founded in 1998, and we have about 220 employees, all focused on aging. And we take great pride in the fact that we started the most simple model, yeast, we go to C. elegans, a nematode, we go to fruit flies, mice, and humans now. And one of the thing that really excites me about the Buck is the fact that we have built this incredible body of knowledge over the last 20 years in terms of the basic biology of aging, and I think the field has moved to the point that we’re really ready to start translating all of these into humans. All of us are seeing, you know, the incredible interest from pharma and venture capital in terms of investing in the aging space. And I think, you know, we’re really well-positioned to be riding that next wave which is going to be bringing all of these incredible discoveries into humans. And so stay tuned.
- Rhonda: I’m certainly gonna stay tuned. Thank you so much for taking time to speak with me today.
- Dr. Verdin: My pleasure, my pleasure. It was great.
Acetyl coenzyme A is a molecule that was first discovered to transfer acetyl groups to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Now it is known to be involved in many different pathways including fatty acid metabolism, steroid synthesis, acetylcholine synthesis, acetylation, and melatonin synthesis.
The death rate from all causes of death for a population in a given time period.
Star-shaped cells found in the brain and spinal cord. Astrocytes facilitate neurotransmission, provide nutrients to neurons, maintain neuronal ion balance, and support the blood-brain barrier. Astrocytes also play a role in the repair and scarring process of the brain and spinal cord following traumatic injuries.
An intracellular degradation system involved in the disassembly and recycling of unnecessary or dysfunctional cellular components. Autophagy participates in cell death, a process known as autophagic dell death. Prolonged fasting is a robust initiator of autophagy and may help protect against cancer and even aging by reducing the burden of abnormal cells.
The relationship between autophagy and cancer is complex, however. Autophagy may prevent the survival of pre-malignant cells, but can also be hijacked as a malignant adaptation by cancer, providing a useful means to scavenge resources needed for further growth.
A bidirectional cell signaling pathway that may regulate cell function, metabolism, or other aspects of physiology. Most signaling pathways are unidirectional. However, an axis may involve two or more signaling proteins and their secreting organs or cells in a type of feedback loop. For example, the growth hormone/IGF axis, also known as the Hypothalamic–pituitary–somatotropic axis, is a highly regulated pathway involving IGF-1 (produced by the liver), growth hormone (produced by the pituitary), and growth hormone-releasing hormone (produced by the hypothalamus).
A chemical produced in the liver via the breakdown of fatty acids. Beta-hydroxybutyrate is a type of ketone body. It can be used to produce energy inside the mitochondria and acts as a signaling molecule that alters gene expression by inhibiting a class of enzymes known as histone deacetylases.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A transparent nematode species (a type of roundworm), about 1mm in length. The first multicellular organism to have its whole genome sequenced. Because they have a short lifespan (about 14-15 days), they are a good model organism for aging research. Strains are inexpensive to breed and can be frozen. When subsequently thawed, they remain viable, allowing long-term storage.
The practice of long-term restriction of dietary intake, typically characterized by a 20 to 50 percent reduction in energy intake below habitual levels. Caloric restriction has been shown to extend lifespan and delay the onset of age-related chronic diseases in a variety of species, including rats, mice, fish, flies, worms, and yeast.
Compounds that induce a similar biochemical milieu in the cell as starvation or nutrient deprivation, including the reductions in cytosolic acetyl CoA and increases in protein deacetylation that serve as a trigger for the cellular autophagic machinery. Popular examples of compounds that exhibit this type of effect include: hydroxycitrate (inhibits ATP citrate lyase), spermidine (inhibits Ep300, a protein acetyltransferase), and resveratrol (activates deacetylases called sirtuins).
A gene encoding a transcription factor (CLOCK) that affects both the persistence and period of circadian rhythms. CLOCK functions as an essential activator of downstream elements in the pathway critical to the generation of circadian rhythms. In humans, polymorphisms in the CLOCK gene have been associated with increased insomnia, weight loss difficulty, and recurrence of major depressive episodes in patients with bipolar disorder.
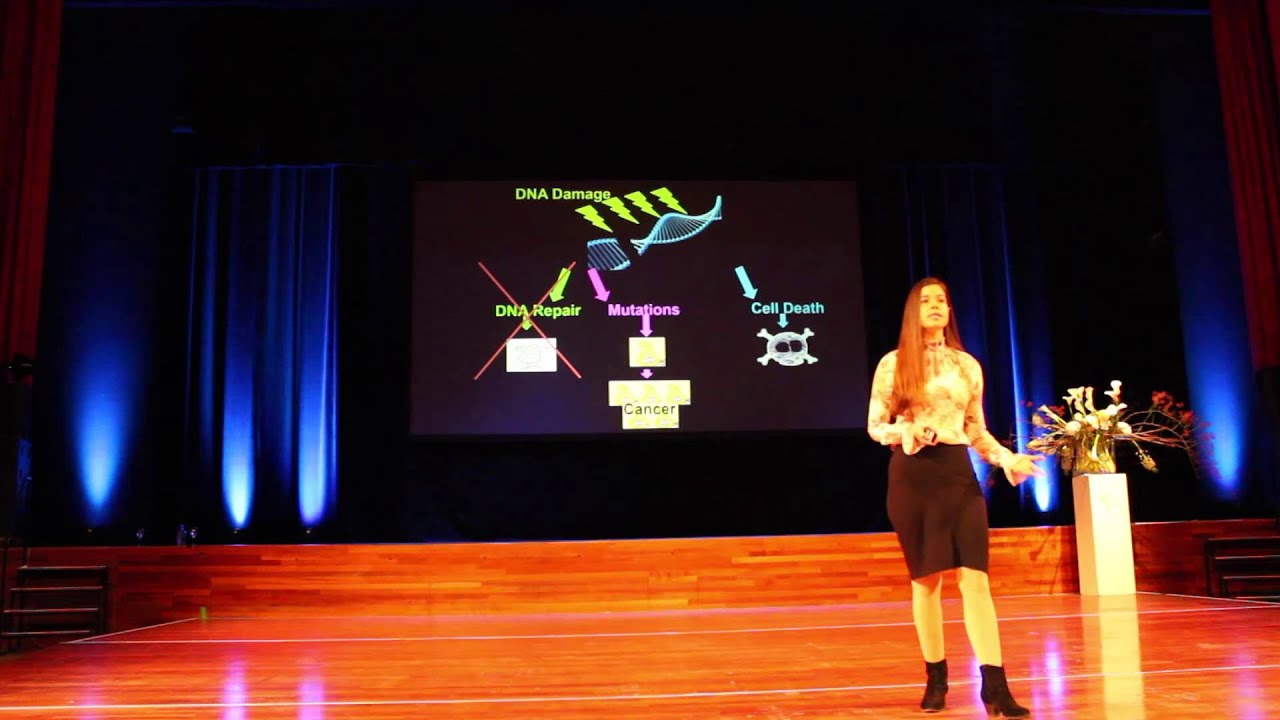
A major contributing factor to aging, cellular senescence, and the development of cancer. Byproducts of both mitochondrial energy production and immune activity are major sources of DNA damage. Additionally, environmental stressors can increase this base level of damage. DNA damage can be mitigated by cellular repair processes; however, the effectiveness of these processes may be influenced by the availability of dietary minerals, such as magnesium, and other dietary components, which are needed for proper function of repair enzymes.
A genus of flies, often called "fruit flies," that has been heavily used in research in genetics and is a common model organism in developmental biology. Fruit flies are popular experimental animals because they are easily cultured en masse out of the wild, have a short generation time, and mutants are readily obtainable.
Any of a group of complex proteins or conjugated proteins that are produced by living cells and act as catalyst in specific biochemical reactions.
Also known as p300 HAT. A histone acetyltransferase that acetylates proteins in chromatin, causing widespread changes in gene activation. This enzyme can be inhibited by compounds such as spermidine and thereby promote autophagy.
A biomarker of aging based on alterations in an organism’s DNA methylation (DNAm) profile. Methylations occur naturally and regulate gene expression. With age, the methylation state of a gene may change. These changes are quantifiable, serving as a means to gauge biological age, which is often different from chronological age. Several variations of epigenetic clocks have been identified. They are generally categorized according to the type and number of tissues used to formulate the calculation, as well as the type of age measured (e.g., epigenetic versus phenotypic). The most widely used clocks include: - HorvathAge, which predicts intrinsic epigenetic age acceleration, a phenomenon in which an organism's aging is influenced by internal physiological factors such as normal metabolism and genetics.[1] - DNAm PhenoAge, which predicts time-to-death among people of the same chronological age, based on biomarkers of age-related disease.[2] - DNAm GrimAge, which predicts lifespan and healthspan, based on DNAm surrogates in blood, including biomarkers of aging and alterations in blood composition.[3]
- ^ Horvath S (2013). DNA methylation age of human tissues and cell types. Genome Biol 14, 10.
- ^ Levine, Morgan E.; Lu, Ake T.; Quach, Austin; Chen, Brian H.; Assimes, Themistocles L.; Bandinelli, Stefania, et al. (2018). An Epigenetic Biomarker Of Aging For Lifespan And Healthspan Aging 10, 4.
- ^ Lu, Ake T.; Quach, Austin; Wilson, James G.; Reiner, Alex P.; Aviv, Abraham; Raj, Kenneth, et al. (2019). DNA Methylation GrimAge Strongly Predicts Lifespan And Healthspan Aging 11, 2.
Genetic control elicited by factors other than modification of the genetic code found in the sequence of DNA. Epigenetic changes determine which genes are being expressed, which in turn may influence disease risk. Some epigenetic changes are heritable.
A diet that mimics the effects of fasting on markers associated with the stress resistance induced by prolonged fasting, including low levels of glucose and IGF-1, and high levels of ketone bodies and IGFBP-1. More importantly, evidence suggests these changes in the cellular milieu are associated with a sensitization of cancer cells to chemotherapeutic drugs while simultaneously also conferring greater stress resistance to healthy cells.[1] Evidence also continues to emerge that properties of the fasting-mimicking diet, particularly its ability to cause immune cell turnover, may also make it useful in the amelioration of auto-immune diseases like multiple sclerosis.[2]
Fasting-Mimicking Diet Breakdown
- Day 1 - consists of 1,090 total calories (10% protein, 55% fat and 34% carbohydrate)
- Days 2 through 5 - consists of 725 total calories (9% protein, 44% fat and 47% carbohydrate)
[1] Cheng, Chia-Wei, et al. "Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression." Cell Stem Cell 14.6 (2014): 810-823. [2] Choi, In Young, et al. "A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms." Cell Reports 15.10 (2016): 2136-2146.
A molecule composed of carboxylic acid with a long hydrocarbon chain that is either saturated or unsaturated. Fatty acids are important components of cell membranes and are key sources of fuel because they yield large quantities of ATP when metabolized. Most cells can use either glucose or fatty acids for this purpose.
A protein that provides the instructions for genes responsible for the regulation of cellular replication, resistance to oxidative stress, metabolism, and DNA repair. FOXO3 may play an integral part in both longevity and tumor suppression. Variants of FOXO3 are associated with longevity in humans. Humans with a more active version of this gene have a 2.7-fold increased chance of living to be a centenarian.
The process in which information stored in DNA is converted into instructions for making proteins or other molecules. Gene expression is highly regulated. It allows a cell to respond to factors in its environment and involves two processes: transcription and translation. Gene expression can be turned on or off, or it can simply be increased or decreased.
A metabolic pathway in which the liver produces glucose from non-carbohydrate substrates including glycogenic amino acids (from protein) and glycerol (from lipids).
A survival mechanism the brain relies on during starvation. Glucose sparing occurs when the body utilizes fatty acids as its primary fuel and produces ketone bodies. The ketone bodies cross the blood-brain barrier and are used instead of glucose, thereby “sparing” glucose for use in other metabolic pathways, such as the pentose-phosphate pathway, which produces NADPH. NADPH is essential for the production of glutathione, one of the major antioxidants used in the body and brain.
An antioxidant compound produced by the body’s cells. Glutathione helps prevent damage from oxidative stress caused by the production of reactive oxygen species.
A highly branched chain of glucose molecules that serves as a reserve energy form in mammals. Glycogen is stored primarily in the liver and muscles, with smaller amounts stored in the kidneys, brain, and white blood cells. The amount stored is influenced by factors such as physical training, basal metabolic rate (BMR), and eating habits.
A series of enzyme-dependent reactions that breaks down glucose. Glycolysis converts glucose into pyruvate, releasing energy and producing ATP and NADH. In humans, glycolysis occurs in the cytosol and does not require oxygen.
The years of a person’s life spent free of disease.
The chief protein components of chromatin found in eukaryotic cell nuclei that package and order the DNA into structural units called nucleosomes acting as spools around which DNA winds, and playing a role in gene regulation.
The gradual deterioration of the immune system brought on by natural age advancement. Immunosenescence is considered the most important reason for the increased rate of infections (and cancers) in older adults and is believed to be the diminished or exhausted function of the immune system that naturally occurs with aging.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A peptide hormone secreted by the beta cells of the pancreatic islets cells. Insulin maintains normal blood glucose levels by facilitating the uptake of glucose into cells; regulating carbohydrate, lipid, and protein metabolism; and promoting cell division and growth. Insulin resistance, a characteristic of type 2 diabetes, is a condition in which normal insulin levels do not produce a biological response, which can lead to high blood glucose levels.
One of the most potent natural activators of the AKT signaling pathway. IGF-1 stimulates cell growth and proliferation, inhibits programmed cell death, mediates the effects of growth hormone, and may contribute to aging and enhancing the growth of cancer after it has been initiated. Similar in molecular structure to insulin, IGF-1 plays a role in growth during childhood and continues later in life to have anabolic, as well as neurotrophic effects. Protein intake increases IGF-1 levels in humans, independent of total caloric consumption.
A broad term that describes periods of voluntary abstention from food and (non-water) drinks, lasting several hours to days. Depending on the length of the fasting period and a variety of other factors, intermittent fasting may promote certain beneficial metabolic processes, such as the increased production of ketones due to the use of stored fat as an energy source. The phrase “intermittent fasting” may refer to any of the following:
- Time-restricted eating
- Alternate-day fasting
- Periodic fasting (multi-day)
A metabolic pathway in which organisms produce ketones. Ketogenesis occurs primarily in the mitochondria of liver cells via the breakdown of fatty acids and ketogenic amino acids. Insulin is the major hormonal regulator of ketogenesis; however, glucagon, cortisol, thyroid hormones, and catecholamines can induce greater breakdown of free fatty acids, thereby increasing the substrates available for use in the ketogenic pathway. The primary ketones used by the body for energy are acetoacetate and beta-hydroxybutyrate.
A diet that causes the body to oxidize fat to produce ketones for energy. A ketogenic diet is low in carbohydrates and high in proteins and fats. For many years, the ketogenic diet has been used in the clinical setting to reduce seizures in children. It is currently being investigated for the treatment of traumatic brain injury, Alzheimer's disease, weight loss, and cancer.
Molecules (often simply called “ketones”) produced by the liver during the breakdown of fatty acids. Ketone production occurs during periods of low food intake (fasting), carbohydrate restrictive diets, starvation, or prolonged intense exercise. There are three types of ketone bodies: acetoacetate, beta-hydroxybutyrate, and acetone. Ketone bodies are readily used as energy by a diverse array of cell types, including neurons.
Lactate is thought to participate in a sort of "lactate shuttle" where, after being produced in muscle from exercise, it is transported in to tissues like the heart, and brain, where it is used as an energy source. Lactate is one of many molecules that falls under a loose group of molecules referred to as exerkines, a broad group of exercise-induced hormonal-like factors. Evidence suggests that lactate is the preferred fuel of the brain. Additionally, rodent studies suggest that lactate mediates some of the benefits of exercise on learning and memory via inducing neuronal brain-derived neurotrophic factor (BDNF) expression.[1] In clinical studies, lactate shows promise as a treatment for inflammatory conditions including traumatic brain injury and as a means to deliver fuel to working muscles.
- ^ Helge, Jørn Wulff; Moritz, Thomas; Morville, Thomas; Clemmensen, Christoffer; Dela, Flemming (2020). Plasma Metabolome Profiling Of Resistance Exercise And Endurance Exercise In Humans Cell Reports 33, 13.
The three basic components of the human diet. Macronutrients are consumed in large quantities and provide necessary energy for the body. They include carbohydrates, fats, and proteins.
An enzyme that participates in genetic pathways that sense amino acid concentrations and regulate cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription. mTOR integrates other pathways including insulin, growth factors (such as IGF-1), and amino acids. It plays key roles in mammalian metabolism and physiology, with important roles in the function of tissues including liver, muscle, white and brown adipose tissue, and the brain. It is dysregulated in many human diseases, such as diabetes, obesity, depression, and certain cancers. mTOR has two subunits, mTORC1 and mTORC2. Also referred to as “mammalian” target of rapamycin.
Rapamycin, the drug for which this pathway is named (and the anti-aging properties of which are the subject of many studies), was discovered in the 1970s and is used as an immunosuppressant in organ donor recipients.
The thousands of biochemical processes that run all of the various cellular processes that produce energy. Since energy generation is so fundamental to all other processes, in some cases the word metabolism may refer more broadly to the sum of all chemical reactions in the cell.
A biochemical process involving the addition or subtraction of a methyl group (CH3) to another chemical group. In epigenetics, a methyl group is added to an amino acid in a histone tail on DNA, altering the activity of the DNA segment without changing its sequence. Under- and over-methylation are referred to as hypomethylation and hypermethylation, respectively.
Vitamins and minerals that are required by organisms throughout life in small quantities to orchestrate a range of physiological functions. The term micronutrients encompasses vitamins, minerals, essential amino acids, essential fatty acids.
Tiny organelles inside cells that produce energy in the presence of oxygen. Mitochondria are referred to as the "powerhouses of the cell" because of their role in the production of ATP (adenosine triphosphate). Mitochondria are continuously undergoing a process of self-renewal known as mitophagy in order to repair damage that occurs during their energy-generating activities.
The process by which new mitochondria are made inside cells. Many factors can activate mitochondrial biogenesis including exercise, cold shock, heat shock, fasting, and ketones. Mitochondrial biogenesis is regulated by the transcription factor peroxisome proliferator-activated receptor gamma coactivator 1-alpha, or PGC-1α.
A coenzyme that is required for the production of energy in cells. NAD+ is synthesized from three major precursors: tryptophan, nicotinic acid (vitamin B3), and nicotinamide. It regulates the activity of several key enzymes including those involved in metabolism and repairing DNA damage. NAD+ levels rise during a fasted state. A group of enzymes called sirtuins, which are a type of histone deacetylase, use NAD+ to remove acetyl groups from proteins and are important mediators for the effects of fasting, caloric restriction, and the effects of the plant compound resveratrol, a so-called caloric restriction mimetic.
A precursor molecule for the biosynthesis of nicotinamide adenine dinucleotide (NAD+), a coenzyme that participates in the production of cellular energy and repair. NMN helps maintain cellular levels of NAD+, thereby facilitating NAD+-dependent cellular activities, such as mitochondrial metabolism, regulation of sirtuins, and PARP activity. Animal studies have demonstrated that NMN administration is effective in increasing NAD+ levels across multiple tissues while improving the outcome of a variety of age-related diseases. Although NMN administration has proven to be safe and to effectively increase NAD+ levels in rodents, the safety and efficacy of NMN supplementation in humans remain unknown. NMN is available in supplement form and is present in various types of food, including broccoli, avocado, and beef. It is also an intermediate compound in the NAD+ salvage pathway, the recycling of nicotinamide into NAD+.
One of four nitrogen-containing molecules that comprise DNA. A nucleotide consists of one of four chemicals, called a “base,” plus one molecule of sugar and one molecule of phosphoric acid. Nucleotides are typically identified by the first letter of their base names: adenine (A), cytosine (C), guanine (G), and thymine (T). They form specific pairs (A with T, and G with C), and their bonds provide the helical structure of the DNA strand.
The process of generating energy that occurs when mitochondria couple oxygen with electrons that have been derived from different food sources including glucose, fatty acids, and amino acids.
A result of oxidative metabolism, which causes damage to DNA, lipids, proteins, mitochondria, and the cell. Oxidative stress occurs through the process of oxidative phosphorylation (the generation of energy) in mitochondria. It can also result from the generation of hypochlorite during immune activation.
An alternate pathway for the oxidation of glucose. The pentose phosphate pathway parallels glycolysis, but does not require or produce ATP; rather, it produces NADPH, which is necessary to create the cellular antioxidant glutathione. Like glycolysis, the pentose phosphate pathway occurs in the cytoplasm.
A family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death (apoptosis). PARP's primary role is to detect and initiate an immediate cellular response to metabolic, chemical, or radiation-induced single-strand DNA breaks by signaling the enzymatic machinery involved in repair. NAD+ is required as substrate for generating ADP-ribose monomers. Evidence suggests that overactivation of PARP may deplete cellular stores of NAD+.
One of the three isotypes of a subfamily of nuclear receptor proteins (the PPARs) that functions as a transcription factor. PPAR-alpha is a major regulator of lipid metabolism in the liver and is activated under conditions of energy deprivation. It is necessary for the process of ketogenesis, a process that is a key adaptive response to prolonged fasting and is inducible by strict carbohydrate restriction. Activation of PPAR-alpha promotes uptake, utilization, and catabolism of fatty acids by upregulation of genes involved in fatty acid transport, fatty acid binding and activation, and peroxisomal and mitochondrial fatty acid β-oxidation. Expression of PPAR-alpha is highest in tissues that oxidize fatty acids at a rapid rate, especially the liver, but also brown adipose tissue (BAT), the heart, and kidney.
A chemical reaction that removes an acetyl functional group from a chemical compound. The presence of the acetyl functional group plays an important role in the synthesis, stability and localization of about 85% of human proteins.[1] During fasting, falling acetyl CoA levels in the cytosol initiate protein deacetylation and initiates autophagy. In general, protein deacetylation, whether from so-called caloric restriction mimetics or nutrient deprivation, is an important general inducer of autophagy.
- ^ Arnesen, Thomas; Van Damme, Petra; Martinho, Rui Gonçalo; Helsens, Kenny; Hole, Kristine; Pimenta-Marques, Ana, et al. (2011). NatF Contributes To An Evolutionary Shift In Protein N-Terminal Acetylation And Is Important For Normal Chromosome Segregation PLOS Genetics 7, 7.
A polyphenolic compound produced in plants in response to injury or pathogenic attack from bacteria or fungi. Resveratrol exerts a diverse array of biological effects, including antitumor, antioxidant, antiviral, and hormonal activities. It activates sirtuin 1 (SIRT1), an enzyme that deacetylates proteins and contributes to cellular regulation (including autophagy). Dietary sources of resveratrol include grapes, blueberries, raspberries, and mulberries.
Resveratrol Autophagy ↑ Deacetylases (especially SIRT1) → ↓ Protein Acetylation → Autophagy
Senescence is a response to stress in which damaged cells suspend normal growth and metabolism. While senescence is vital for embryonic development, wound healing, and cancer immunity, accumulation of senescent cells causes increases inflammation and participates in the phenotype of aging.
A molecule that allows cells to perceive and correctly respond to their microenvironment, which enables normal cellular function, tissue repair, immunity, cognition, and more. Hormones and neurotransmitters are examples of signaling molecules. There are many types of signaling molecules, however, including cAMP, nitric oxide, estrogen, norepinephrine, and even reactive oxygen species (ROS).
A class of enzymes that influence that influence aging and longevity through multiple molecular pathways. Sirtuins regulate a variety of metabolic processes, including release of insulin, mobilization of lipids, response to stress, and modulation of lifespan. They also influence circadian clocks and mitochondrial biogenesis. Sirtuins are activated when NAD+ levels rise. The dependence of sirtuins on NAD+ links their enzymatic activity directly to the energy status of the cell via the cellular NAD+:NADH ratio, the absolute levels of NAD+, NADH or nicotinamide or a combination of these variables. There are seven known sirtuins, designated as Sirt1 to Sirt7.
A cell that has the potential to develop into different types of cells in the body. Stem cells are undifferentiated, so they cannot do specific functions in the body. Instead, they have the potential to become specialized cells, such as muscle cells, blood cells, and brain cells. As such, they serve as a repair system for the body. Stem cells can divide and renew themselves over a long time. In 2006, scientists reverted somatic cells into stem cells by introducing Oct4, Sox2, Klf4, and cMyc (OSKM), known as Yamanaka factors.[1]
- ^ Takahashi, Kazutoshi; Yamanaka, Shinya (2006). Induction Of Pluripotent Stem Cells From Mouse Embryonic And Adult Fibroblast Cultures By Defined Factors Cell 126, 4.
Distinctive structures comprised of short, repetitive sequences of DNA located on the ends of chromosomes. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division. When the telomeres on a cell’s chromosomes get too short, the chromosome reaches a “critical length,” and the cell stops dividing (senescence) or dies (apoptosis). Telomeres are replenished by the enzyme telomerase, a reverse transcriptase.
A protein that binds to specific DNA sequences, thereby controlling the rate of transcription of genetic information from DNA to messenger RNA. A defining feature of transcription factors is that they contain one or more DNA-binding domains, which attach to specific sequences of DNA adjacent to the genes that they regulate.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.