Sulforaphane and its byproducts may protect against bladder cancer | Jed Fahey
Enter your email to get our 15-page guide to sprouting broccoli and learn about the science of chemoprotective compount sulforaphane.
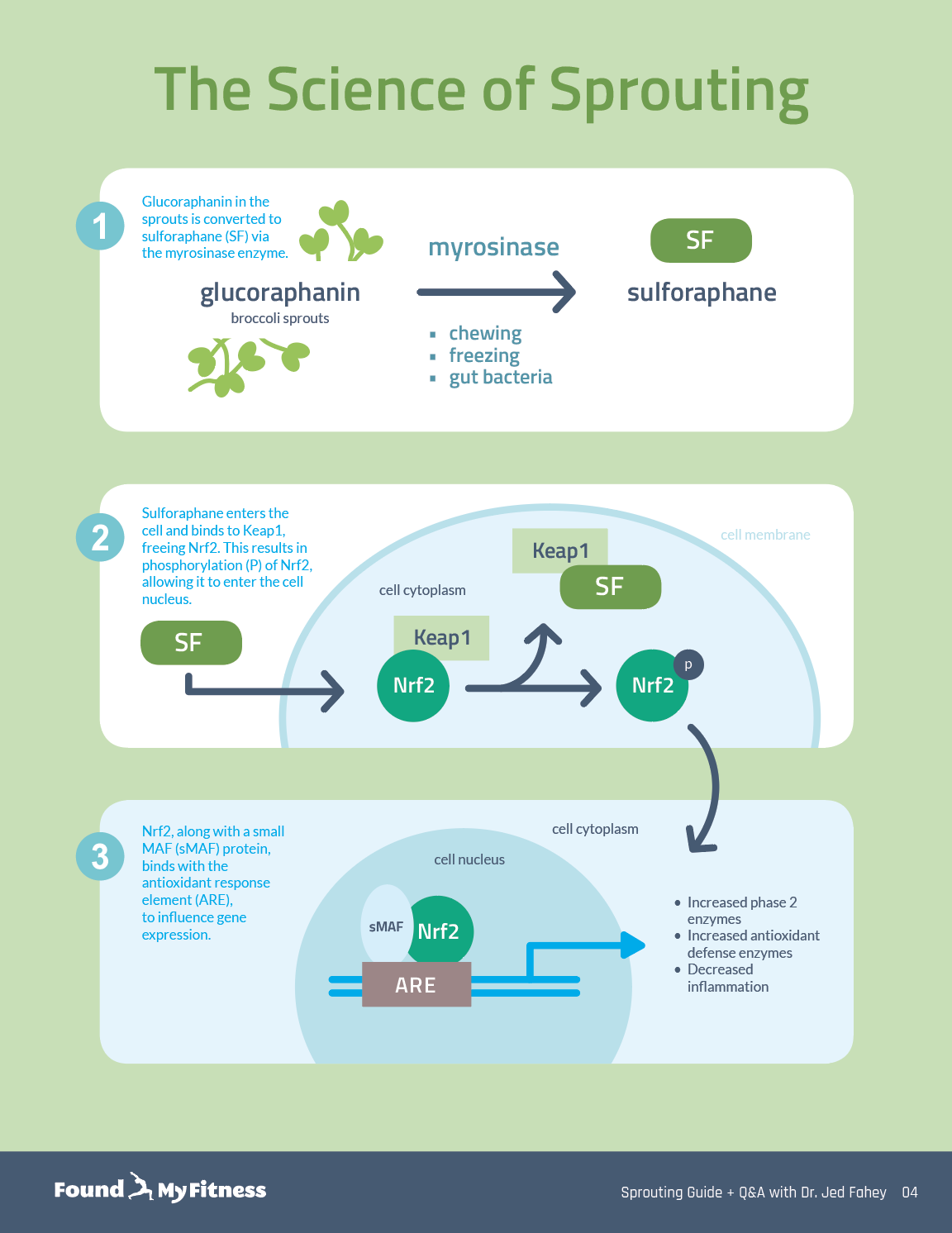
Broccoli sprouts are concentrated sources of sulforaphane, a type of isothiocyanate. Damaging broccoli sprouts – when chewing, chopping, or freezing – triggers an enzymatic reaction in the tiny plants that produces sulforaphane.
Get the full length version of this episode as a podcast.
This episode will make a great companion for a long drive.
The bladder serves as a reservoir for byproducts of sulforaphane metabolism, including a variety of glutathione-derived conjugates. These antioxidant compounds eventually end up in the urine, which is temporarily stored in the bladder prior to elimination, providing a unique environment in which to study the effects of sulforaphane and its metabolic byproducts on bladder tissue. Although human trials are lacking, some animal research suggests that sulforaphane and its byproducts protect against bladder cancer. In this clip, Dr. Jed Fahey discusses the effects of urinary excretion of sulforaphane byproducts.
- [Dr. Patrick]: And just to add on as a comment, you know, speaking to prevention. I know there's been...there's... Some of the preclinical evidence that really excited me was there was a rat study. And I forget the group that published the study. But the rats were given sulforaphane, they were given it before they were given a chemical that causes bladder cancer. And the other group of rats were just given the chemical that causes bladder cancer. And the rats that were given the dose of the sulforaphane did not develop bladder cancer. And if they did, the tumors were much, much smaller in size. So again, preventative. You know, just more evidence that the preventative approach seems to be stronger.
There's also been the clinical evidence. It seems as though... And your...and you know better about this, Jed, about, you know, the tissues that sulforaphane may accumulate in more, you know, the bladder, prostate, breast, versus other tissues, or lungs. You know, I don't even know if we know all the differences. But the prostate seems to be one that at least clinically there's been a few studies showing that sulforaphane, ranging in dose, the higher the dose the more robust the effect. So 60 milligrams of sulforaphane a day was shown to slow the biomarker prostate-specific antigen, or PSA, which people with, you know, early prostate cancer or, you know, a risk of it or whatever have. And so slowing that by 86% is certainly, you know, a good thing.
[Dr. Fahey]: Yeah. So as a follow-up, shame on you, I think that first bladder cancer study you were talking about is also one that I was involved in.
[Dr. Patrick]: Okay, so at the beginning of the call I said I'd been following your work because you're every study. So pretty much every study that I cite almost Jed Fahey, Dr. Jed Fahey, is an author on. So it's just you're so prolific, I can't keep track of all your publications, Jed.
[Dr. Fahey]: Mostly a minor player. So in this case a minor player. A group in New Zealand, Rex Munday and an old, old colleague, actually the guy who, with Paul Talalay, put sulforaphane on the map, Yingsha Zhang, and I did this...did one of the rat studies, I think maybe the one you're talking about, with bladder cancer. And absolutely, this was benzyl isothiocyanate, as I recall, that we used, or sinigrin, allyl isothiocyanate. Which come from radish or daikon mustard seed.
So it's worth dwelling on this. When you think about what happens with sulforaphane, which most of you...I don't know, most of you don't. I do probably too much, to my detriment. But what happens? You ingest it. You take it in, the body sees it, your cells of your body see it, they actually take it in very rapidly, they conjugate it with glutathione, and they spit it back out. Then where does it go? It goes from the blood to the urine. Then where does the urine go? Unless you're on a Foley catheter, and most of us that are reasonable healthy aren't, although I've had that fun experience before. So your bladder stores urine up. What's in that urine? Sulforaphane and its conjugates. Well, what is bladder cancer? Bladder cancer is the epithelial cells of the bladder, most of them, the tissue lining that bladder, you know, a mutation forming and ultimately a tumor developing.
So bladder cancer, I still think, is the ultimate target for isothiocyanates because you're bathing that tissue in these protective compounds, essentially all the time. You know, you empty your bladder and immediately it starts filling back up again.
Certainly the prostate cancer is another example where mechanistically, or I should say "evidentiarily," we have...there's evidence. The cause and effect is a little harder to see, but the bladder cancer model is just beautiful.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
An antioxidant compound produced by the body’s cells. Glutathione helps prevent damage from oxidative stress caused by the production of reactive oxygen species.
An essential mineral present in many foods. Iron participates in many physiological functions and is a critical component of hemoglobin. Iron deficiency can cause anemia, fatigue, shortness of breath, and heart arrhythmias.
Byproduct of a reaction between two compounds (glucosinolates and myrosinase) that are found in cruciferous vegetables. Isothiocyanates inhibit phase I biotransformation enzymes, a class of enzymes that transform procarcinogens into their active carcinogenic state. Isothiocyanates activate phase II detoxification enzymes, a class of enzymes that play a protective role against DNA damage caused by reactive oxygen species and carcinogens. Examples of phase II enzymes include UDP-glucuronosyltransferases, sulfotransferases, N-acetyltransferases, glutathione S-transferases, and methyltransferases.
An isothiocyanate compound derived from cruciferous vegetables such as broccoli, cauliflower, and mustard. Sulforaphane is produced when the plant is damaged when attacked by insects or eaten by humans. It activates cytoprotective mechanisms within cells in a hormetic-type response. Sulforaphane has demonstrated beneficial effects against several chronic health conditions, including autism, cancer, cardiovascular disease, diabetes, and others.
Attend Monthly Q&As with Rhonda
Support our work

The FoundMyFitness Q&A happens monthly for premium members. Attend live or listen in our exclusive member-only podcast The Aliquot.





































































