Nicotinamide mononucleotide Suggest an improvement to this article
Nicotinamide mononucleotide (NMN) is a precursor molecule for the biosynthesis of nicotinamide adenine dinucleotide, or NAD+, a coenzyme that participates in the production of cellular energy and DNA repair. NAD+ can also be synthesized from other precursors including nicotinamide (NAM, also called niacinamide), nicotinic acid (NA), and nicotinamide riboside (NR), which are often collectively referred to as niacin (vitamin B3) or niacin equivalents.[1] These precursors are not equally allocated throughout the body; rather, they exhibit preferential distribution among the blood, brain, gut, and other organs. Tryptophan, an amino acid obtained from the diet, can also be converted into NAD+.
Nicotinamide mononucleotide is present in various types of food, including broccoli, avocado, and beef, but it is also an intermediate compound in the NAD+ salvage pathway, the recycling of nicotinamide into NAD+.
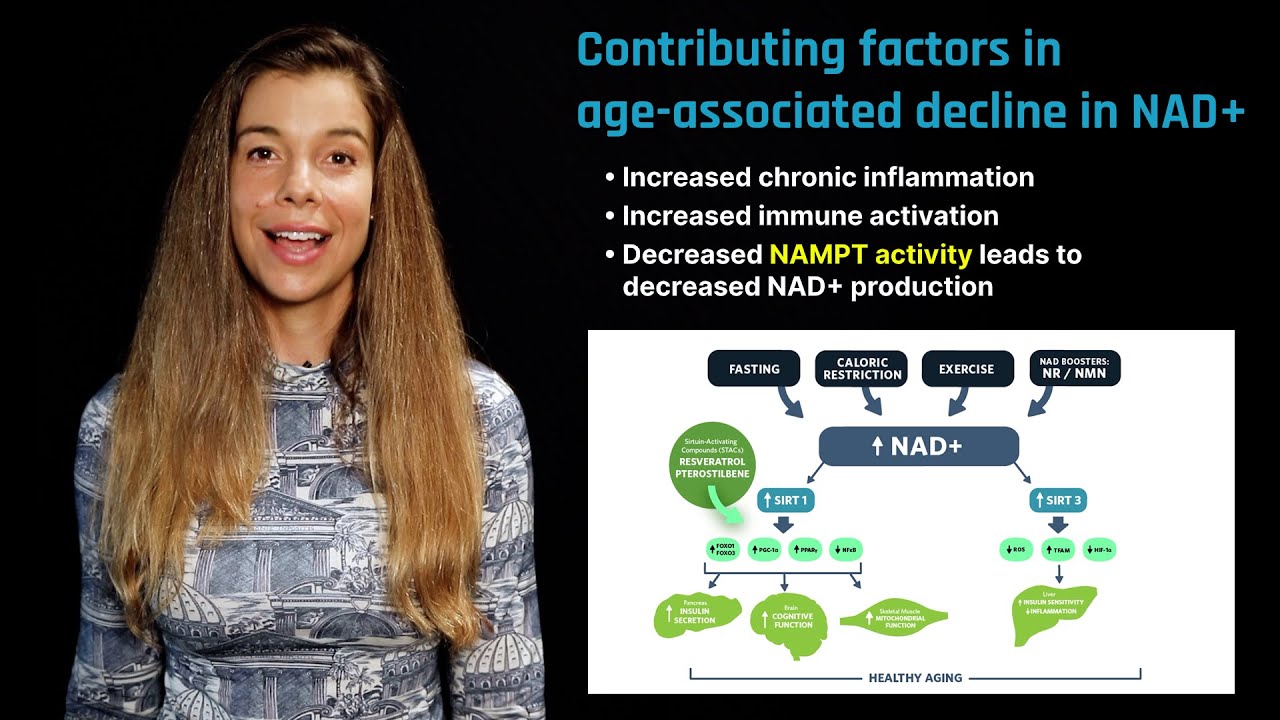
NAD+ acts as a cellular "sensor" to mediate energy-generating pathways and activate NAD+-consuming enzymes such as sirtuins and poly ADP-ribose polymerases (PARP).[2] [3] NAD+ depletion, which occurs with age, has been implicated in the progression of multiple age-related conditions such as metabolic dysregulation and neurodegenerative diseases. Nicotinamide mononucleotide, as well as other precursors such as NR, help maintain cellular levels of NAD+. Boosting NAD+ levels through precursors has proven to have therapeutic potential in treating aging and age-related diseases across multiple animal models. The first human phase 1 clinical trial is now underway to determine the safety and bioavailability of nicotinamide mononucleotide supplementation.[4]
Learn more about NAD+ in this overview article.
Beneficial health effects
Animal studies have shown nicotinamide mononucleotide administration to be effective in increasing NAD+ levels across multiple tissues while improving the outcome of a variety of age-related diseases in multiple rodent studies.
Obesity and type 2 diabetes
Insulin resistance is a major feature of type 2 diabetes, with obesity and aging acting as major contributors in the pathophysiology of this disease. In a study involving diet-induced diabetic mice, injection of 500 milligrams per kilogram of body weight (mg/kg/bw) of nicotinamide mononucleotide administered for seven days enhanced insulin sensitivity and improved glucose intolerance compared to that observed among mice not given nicotinamide mononucleotide.[5] These effects may be due to the ability of nicotinamide mononucleotide to elicit anti-inflammatory effects in dysfunctional pancreatic beta cells, the site of insulin synthesis and secretion.[6] Similar results were observed when female diet-induced obese mice injected with 500 mg/kg/bw of nicotinamide mononucleotide for 17 days exhibited increased NAD+ levels in the liver and muscle and improved glucose tolerance compared to mice fed an obesigenic diet alone.[7]
Cardiovascular health
Cardiovascular disease is the leading cause of death in the United States. Ischemia, the reduction of blood flow to the heart, followed by reperfusion, the restoration of blood flow, are life-threatening events. Heart damage induced by ischemia/reperfusion is an important cause of heart failure.[8] Although human trials are lacking, nicotinamide mononucleotide administration in rodent models improved cardiovascular function and provided protection against ischemia/reperfusion injury. A study in male mice found that 500 mg/kg/bw injection of nicotinamide mononucleotide administered 30 minutes before and four times after ischemia protected the heart from reperfusion injury.[9] In a study in which old mice, 26 to 28 months of age, were administered 300 mg/kg/bw of nicotinamide mononucleotide in their diet for 8 weeks, age‐associated arterial dysfunction and oxidative stress were reversed and arterial SIRT1 activity was increased.[10] In addition, in a mouse model of cardiomyopathy – a disease of the heart muscle that hinders the pumping of blood to the rest of the body – injection of 500 mg/kg/bw of nicotinamide mononucleotide twice weekly for six weeks improved cardiac contractility compared to that observed in mice not given NMN.[11]
Treatment of neurodegenerative disease
Nicotinamide mononucleotide administration has also been shown to improve cognition and memory in mouse and rat models of Alzheimer’s disease. Rats that were injected with 500 mg/kg/bw of nicotinamide mononucleotide every day for 10 days demonstrated sustained improvement in cognitive function as well as an attenuation of neuronal cell death.[12] Furthermore, nicotinamide mononucleotide administered at 100 mg/kg/bw for 28 days to mice genetically predisposed to develop Alzheimer's disease elicited substantial improvements in behavioral measures of cognitive impairment compared to mice not given NMN. Additionally, nicotinamide mononucleotide treatment significantly decreased beta-amyloid production and inflammatory responses, both of which may be mediators of Alzheimer's disease progression.[13]
Aging and longevity
Decreasing levels of NAD+ are observed with aging and are associated with the development of age-related diseases. A long-term study found that dietary administration of nicotinamide mononucleotide mitigated the age-associated physiological decline in mice during normal aging. Specifically, starting at 5 months of age, mice were fed either 100 or 300 mg/kg/bw nicotinamide mononucleotide for 12 months, which resulted in a dose-dependent reduction in body weight of 4 percent and 9 percent, respectively, compared to mice not given NMN.[14] Additionally, the mice that were fed nicotinamide mononucleotide had improved skeletal muscle mitochondrial function, increased energy expenditure, increased bone density, and decreased insulin resistance in a dose-dependent manner.[14]
DNA damage is thought to be one of the primary drivers of aging. A study in old mice demonstrated that nicotinamide mononucleotide administration can stimulate repair of DNA damage through PARP1. Specifically, after 22-month-old mice were injected with 500 mg/kg/bw of NMN every day for one week, their hepatic NAD+ concentration and PARP1 activity were increased.[15]
Mitochondrial health
Mitochondrial dysfunction is a hallmark of aging, and maintaining mitochondrial integrity and function may be a promising therapy to slow or reverse age-related conditions such as Alzheimer's disease, frailty, or heart failure. In a mouse model of Alzheimer's disease, in which mitochondrial dysfunction is commonly observed, mice that were injected with 100 mg/kg/bw of nicotinamide mononucleotide every other day for 28 days had improved brain mitochondria respiratory capacity.[16] Age-related declines in skeletal muscle mass and function contribute to frailty in older adults. However, long-term administration of nicotinamide mononucleotide in the drinking water of mice for 12 months prevented age-associated gene expression changes in peripheral tissues and enhanced mitochondrial respiratory capacity in skeletal muscle.[14] In heart failure, hyperacetylation of mitochondria is associated with disease progression. Nicotinamide mononucleotide administered at 500 mg/kg/bw once every three days for approximately five weeks in mice reversed mitochondrial protein hyperacetylation and delayed development of heart failure.[17]
Cancer concerns
Nicotinamide phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in the NAD+ salvage pathway that converts nicotinamide to nicotinamide mononucleotide. Both NAMPT and nicotinamide mononucleotide might have pro-angiogenic activity (the capacity to build blood vessels) and support the growth of some types of cancers. For example, in a mouse mammary carcinoma model, pharmacologically inhibiting NAMPT delayed tumor growth with a dose-dependent decrease in NAD+levels.[18]
Furthermore, NAMPT and nicotinamide mononucleotide have been associated with cellular senescence – the growth arrest of cells – which may lead to aging and cancer. In a mouse model of pancreatic cancer, proinflammatory senescent cells drive tumor growth. When mice were injected with 500 mg/kg/bw of nicotinamide mononucleotide for 13 days, they exhibited significant increases in precancerous and cancerous lesions in the pancreas. Conversely, treatment with a NAMPT inhibitor suppressed proinflammatory and senescent markers while decreasing precancerous and cancerous lesions.[19]
While the studies mentioned above suggest a role for NAD+ metabolism in cancer progression, more studies are needed to assess the role of NAD+ and its precursor molecules in various types of cancer. Thus, augmenting diet with supplements such as nicotinamide mononucleotide to increase NAD+ levels may need to be administered with precision to balance any anti-aging effects with potential harmful pro-tumorigenic effects.
Bioavailability
Evidence from multiple rodent studies demonstrated that nicotinamide mononucleotide delivered orally at a high dose (300 mg/kg/bw) can effectively increase levels of NAD+ across multiple tissues, including the pancreas, liver, and skeletal muscle, within 10 to 30 minutes of administration.[5] [9] [14] [20] [21] When a low dose of nicotinamide mononucleotide (50mg/kg/bw) is orally administered to animals, very little NAD+ is found in tissues other than the liver, and all of the NAD+ is derived from the salvage pathway and not directly from NMN.[22] NAD+ derived from the salvage pathway is subject to feedback inhibition by NAD+ levels.
Intravenous administration of nicotinamide mononucleotide delivered intact nicotinamide mononucleotide, which was directly converted to NAD+ into liver, kidney, and muscle tissue in a dose-dependent manner. Nicotinamide mononucleotide did not cross the blood-brain barrier but did raise brain levels of NAD+ derived from the salvage pathway. These data suggest that if nicotinamide mononucleotide is administered intravenously, it can directly form NAD+ and not be subject to feedback inhibition, possibly increasing tissue NAD+ levels to much higher levels than they otherwise would be.
Learn more about NAD+ flux in this overview article.
Supplementation
Nicotinamide mononucleotide supplements are readily available on the market. Although nicotinamide mononucleotide administration has proven to be safe and to effectively increase NAD+ levels in rodents, the safety and efficacy of NMN supplementation in humans remain unknown. However, the first phase 1 clinical trial is underway to determine the safety and bioavailability of nicotinamide mononucleotide in humans.[4]
Conclusion
NAD+ levels decrease with age and may in part drive the aging process and the development of age-related diseases. Boosting NAD+ levels through nicotinamide mononucleotide administration has proven to be effective in improving age-related diseases such as cardiovascular disease and Alzheimer's disease in rodents. The first ongoing human clinical trial should provide insight into the safety and bioavailability of nicotinamide mononucleotide supplementation in humans.
- ^ Imai, Shin-ichiro; Johnson, Sean (2018). NAD+ Biosynthesis, Aging, And Disease F1000Research 7, .
- ^ Kozako, Tomohiro; Hayashida, Satoru; Arimoto, Akie; Kuramoto, Yukako; Honda, Shin-ichiro; Shimeno, Hiroshi, et al. (2010). Fasting Promotes The Expression Of SIRT1, An NAD+-dependent Protein Deacetylase, Via Activation Of PPARα In Mice Molecular And Cellular Biochemistry 339, 1-2.
- ^ Costford, Sheila R.; Pasarica, Magdalena; Albarado, Diana C.; Thomas, Shantele C.; Xie, Hui; Church, Timothy S., et al. (2010). Skeletal Muscle NAMPT Is Induced By Exercise In Humans American Journal Of Physiology-Endocrinology And Metabolism 298, 1.
- ^ a b Tsubota K (2016). The first human clinical study for NMN has started in Japan. NPJ Aging Mech Dis 2, .
- ^ a b Yoshino, Jun; Mills, Kathryn F.; Yoon, Myeong Jin; Imai, Shin-ichiro (2011). Nicotinamide Mononucleotide, A Key NAD+ Intermediate, Treats The Pathophysiology Of Diet- And Age-Induced Diabetes In Mice Cell Metabolism 14, 4.
- ^ Caton, P. W.; Kieswich, J.; Yaqoob, M. M.; Holness, M. J.; Sugden, M. C. (2011). Nicotinamide Mononucleotide Protects Against Pro-Inflammatory Cytokine-Mediated Impairment Of Mouse Islet Function Diabetologia 54, 12.
- ^ Youngson, Neil; Uddin, Golam Mezbah; Sinclair, David A.; Morris, Margaret (2016). Head To Head Comparison Of Short-Term Treatment With The NAD+ Precursor Nicotinamide Mononucleotide (NMN) And 6 Weeks Of Exercise In Obese Female Mice Frontiers In Pharmacology 7, .
- ^ 10.1056/nejmra071667
- ^ a b Sadoshima, Junichi; Yamamoto, Takanobu; Byun, Jaemin; Zhai, Peiyong; Ikeda, Yoshiyuki; Oka, Shinichi (2014). Nicotinamide Mononucleotide, An Intermediate Of NAD+ Synthesis, Protects The Heart From Ischemia And Reperfusion Plos One 9, 6.
- ^ Picciotto, Natalie E.; Gano, Lindsey B.; Johnson, Lawrence C.; Sindler, Amy L.; Mills, Kathryn F.; Imai, Shin-ichiro, et al. (2016). Nicotinamide Mononucleotide Supplementation Reverses Vascular Dysfunction And Oxidative Stress With Aging In Mice Aging Cell 15, 3.
- ^ Martin, Angelical S.; Abraham, Dennis M.; Bhatt, Dhaval P.; Mao, Lan; Cui, Huaxia; Liu, Juan, et al. (2017). Nicotinamide Mononucleotide Requires SIRT3 To Improve Cardiac Function And Bioenergetics In A Friedreich’s Ataxia Cardiomyopathy Model JCI Insight 2, 14.
- ^ Wang, Xiaonan; Hu, Xuejun; Yang, Yang; Takata, Toshihiro; Sakurai, Takashi (2016). Nicotinamide Mononucleotide Protects Against Β-Amyloid Oligomer-Induced Cognitive Impairment And Neuronal Death Brain Research 1643, .
- ^ Yao, Zhiwen; Yang, Wenhao; Gao, Zhiqiang; Jia, Peng (2017). Nicotinamide Mononucleotide Inhibits JNK Activation To Reverse Alzheimer Disease Neuroscience Letters 647, .
- ^ a b c d Mills, Kathryn F.; Yoshida, Shohei; Stein, Liana R.; Grozio, Alessia; Kubota, Shunsuke; Sasaki, Yo, et al. (2016). Long-Term Administration Of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline In Mice Cell Metabolism 24, 6.
- ^ Moniot, Sébastien; Qin, Bo; Lou, Zhenkun; Steegborn, Clemens; Sinclair, D A; Aravind, L., et al. (2017). A Conserved NAD + Binding Pocket That Regulates Protein-Protein Interactions During Aging Science 355, 6331.
- ^ Owens, Katrina; Schlappal, Anna E; Kristian, Tibor; Fishman, Paul S; Schuh, Rosemary A; Long, Aaron N (2015). Effect Of Nicotinamide Mononucleotide On Brain Mitochondrial Respiratory Deficits In An Alzheimer’s Disease-Relevant Murine Model BMC Neurology 15, 1.
- ^ 27489254
- ^ 10.1158/1078-0432.ccr-04-1399
- ^ Nacarelli, Timothy; Lau, Lena; Fukumoto, Takeshi; Zundell, Joseph; Fatkhutdinov, Nail; Aird, Katherine M., et al. (2019). NAD+ Metabolism Governs The Proinflammatory Senescence-Associated Secretome Nature 21, 3.
- ^ Stein, L. R.; Imai, S.-i. (2014). Specific Ablation Of Nampt In Adult Neural Stem Cells Recapitulates Their Functional Defects During Aging The EMBO Journal , .
- ^ Nakagawa, Takashi; Yoshida, Mitsukuni; Yoon, Myeong Jin; Johnson, Sean; Takikawa, Akiko; Usui, Isao, et al. (2015). SIRT1-Mediated eNAMPT Secretion From Adipose Tissue Regulates Hypothalamic NAD+ And Function In Mice Cell Metabolism 21, 5.
- ^ White, Eileen; Su, Xiaoyang; Quinn Iii, William J; Liu, Ling; Hui, Sheng; Krukenberg, Kristin, et al. (2018). Quantitative Analysis Of NAD Synthesis-Breakdown Fluxes Cell Metabolism 27, 5.
Topics related to Aging
-
FOXO
FOXO proteins are transcriptional regulators that play an important role in healthy aging. Some FOXO genes may increase lifespan.
-
Sirtuins
Sirtuins play a key role in healthspan and longevity by regulating a variety of metabolic processes implicated in aging.
-
NAD+
NAD+ is a cofactor that plays an essential role in metabolism, DNA repair, and immunity. Its depletion accelerates aging.
-
Nicotinamide riboside
Nicotinamide riboside is a precursor of NAD+, a coenzyme necessary for energy production and cellular repair. It is available from food and supplements.
-
Metformin
Metformin, a drug commonly used to treat type 2 diabetes, may modulate certain aging processes.
-
Caloric restriction
Caloric restriction is the practice of long-term reduced energy intake. It delays the onset of age-related chronic diseases in animals.