#2 Dr. George Brooks on Lactate Shuttle Theory, Relevance for Traumatic Brain Injury, & More
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
George A. Brooks, PhD, is a professor in the Department of Integrative Biology at the University of California, Berkeley. He has spent more than four decades investigating energy substrate use in humans. His research has led to a greater understanding of the role and metabolic disposition of lactate and was instrumental in identifying the metabolic pathway known as the “lactate shuttle.”
Dr. Brooks’ current research is aimed at identifying treatments for individuals suffering from injuries and infections associated with lactic acidosis, such as traumatic brain injury, heart failure, inflammatory conditions, and HIV infection.
Dr. Brooks earned his master’s and doctorate degrees in exercise physiology at the University of Michigan. He has authored numerous peer-reviewed articles in the field of exercise physiology.
Debunking a long-held myth
Nearly 40 years ago, as a young, intercollegiate athlete, Dr. George Brooks sought answers to his questions about how to improve his athletic performance. That early interest spawned a lifelong career dedicated to debunking what is possibly one of the greatest misunderstandings in exercise physiology – the lactic acid myth.
This myth is based on the commonly held belief that lactate is a waste product formed by working muscles during intense exercise. According to this belief, lactate and its conjugate, lactic acid, build up in muscles, contributing to fatigue and subsequent performance losses. Much of the misunderstanding about lactate arose from early experiments in non-oxygenated frog muscles in which lactate concentrations rose markedly during stimulation. The myth was born, and it perpetuates today among coaches, athletes, and just about everyone else.
However, these dated ideas run counter to new evidence that suggests oral intake of lactate actively improves exercise performance. Other data demonstrate the role lactate plays as an energy substrate and point to uncertainty that proton accumulation, often associated with high lactate concentrations in blood, is a major cause of fatigue.
Another element of the myth centers around the difference between lactic acid and lactate. But this difference mostly serves to distract. What’s important to remember is that, at physiological pH, the majority of lactic acid is in the form of lactate, but the terms can be understood to be mostly interchangeable.
The lactate shuttle hypothesis
"Our unique contribution was to find that not only are [lactate transporters] in the plasma membranes of muscles and heart and other tissues, but they are also in the mitochondria."- Dr. George Brooks Click To Tweet
Lactate is a natural byproduct of glycolysis – the breakdown of sugar. It’s an essential fuel that cells in the heart, liver, and brain rely on. When produced during exercise (especially high intensity workouts like cycling, which shifts metabolism toward glycolysis to more quickly meet the high energetic demands), lactate switches on more than 600 genes involved in muscle adaptation, stimulates mitochondrial biogenesis – the process of making new mitochondria – and promotes protein synthesis, which is essential for muscle growth. The diverse nature of the signaling quality of lactate has led to Dr. Brooks’s coining of the term “lactormone.”
A key element in this process is a metabolic pathway called the lactate shuttle – initially posited by Dr. Brooks – which describes the movement of lactate within and between cells so it can be used. Moreover, more than merely cell to cell, this network of metabolic activity that includes the generation, transport, and consumption of lactate actually spans multiple organ systems: muscle to liver, muscle to brain, muscle to heart. When a person actively uses their muscles in exercise, we now know, transporters in distant tissues like the heart upregulate in direct proportion to the exertion as measured through muscular contraction – providing support for the idea that high heart-rate, lactate-threshold training provides a means of taking advantage of the lactate shuttle. This is the same sort of training you might expect to get from a professional coach, by the way.
Shuttling lactate to the brain
"I often talk to people about using the talk test. So, if we were on two treadmills here when we were having this conversation, and we got going faster and faster, we would get to the point where we couldn't talk anymore." - Dr. George Brooks Click To Tweet
But lactate appears to be even more complex – and essential – than first believed. Lactate serves as a signaling molecule in the brain, where it stimulates the production of neurotransmitters that promote focus and attention, as well as brain-derived neurotrophic factor, known for its role in supporting neurogenesis. And burgeoning research is pointing to the key role lactate may play in treating brain trauma, using lactate infusions that not only bypass the gut but also spare glucose for other immediate needs.
Providing nutrients – especially glucose – to an individual with a brain injury is a standard of care. However, as explained by Dr. Brooks in this episode, glycolysis is impaired in the injured brain. Without glycolysis, lactate production is halted, and the brain, which prefers lactate as a fuel, starves. Brooks and his colleagues are looking at ways to provide lactate to the injured brain to ameliorate the deleterious effects of trauma.
Learn more about Dr. George A. Brooks
- UC Berkeley Integrative Biology Faculty Profile
- Dr. George Brooks - Exercise Physiology Lab
- MDFlux.com
People mentioned
- A. V. Hill
- Jack Azevedo
- Mark Shigenaga
- Mike Horning
- Neil Martin
- Otto Meyerhof
- Paul Vespa
- Raja Hussein
- Thomas Glenn
Relevant links
- Lactic Acid Not Athlete's Poison, But An Energy Source -- If You Know How To Use It (via ScienceDaily)
- Traumatic Brain Injury: Neuroscientists Challenge Conventional Treatment (via ScienceDaily)
- Lactic Acid Is Not Muscle's Foe, It's Fuel (via NYTimes)
- Lactate shuttle may fuel the injured brain (via Physorg)
-
Rhonda introduces Dr. George Brooks, a professor in the Department of Integrative Biology at UC Berkeley; the Director of the Exercise Physiology lab; and pioneer of the lactate shuttle theory.
-
Dr. Brooks begins by telling us a little about his personal story. As a young intercollegiate athlete, Brooks’ performance was suffering. His coach told him it because he had too much lactic acid and too much oxygen debt. This intuitively didn't make sense Brooks, so he sought answers to his questions – sparking a lifelong career in the study of lactate and its physiological roles.
-
Lactate builds up when it isn't being utilized efficiently enough.
-
The Brooks lab was the first to identify the role and location of lactate transporters – found not only in the plasma membranes of cells, but also in the mitochondria, where they do most of their work.
-
The historical background behind the misunderstanding about lactate and its relationship to muscle fatigue arose from experiments by Otto Meyerhof.
-
One classic training adaptation to exercise is to have lower circulating lactate despite a similar degree of effort. In other words, trained athletes clear lactate from their muscles more efficiently, but they also use it as a fuel in the muscles and in other cells. It’s a key gluconeogenic precursor.
-
Physical training increases and can even double the mass of the mitochondrial network.
-
Exercise also doubles the number of lactate transporters.
-
Some cells are especially capable of producing lactate, whereas others are particularly adept at utilizing it after it.
-
*Relevance for training * Most athletes benefit by building an aerobic base early in season, training towards a specific activity. Muscle cells then adapt by stimulating mitochondrial biogenesis due to the presence of lactate.
-
Lactate threshold training – a type of training that focuses on incrementally increasing the exertion threshold at which you begin to build up excess lactate. This usually means training just below the threshold most of the time and then occasionally crossing that threshold.
-
Ways to track training: measure heart rate, directly measure blood lactate.
-
An easy metric for having crossed the lactate threshold is the "talk test.” If you're physically unable to speak (from being out of breath), this means you've probably built up a lot of blood lactate because oxygen is necessary for lactate to be used and disposed of by the mitochondria.
-
Lactate versus "lactic acid" and lactic acid as a misnomer.
-
Polylactate – a substance used in a sports drink he developed known as Cytomax. Supplemental salts of lactate actually alkalize the blood, resolving acidosis and reducing swelling.
-
A discussion of Dr. Brooks’ recommendation of one hour of physical activity per day.
-
Lactate stimulates brain-derived neurotrophic factor, or BDNF, which is necessary for stimulating the growth of new brain cells.
-
Lactate stimulates norepinephrine release from neurons in the locus coeruleus brain region. Norepinephrine helps with focus and attention and is a target for a variety of drugs called norepinephrine reuptake inhibitors.
-
The blood-brain barrier has an abundance of lactate transporters, which allows lactate to pass into the brain. Because lactate moves down a concentration gradient, it can be delivered into the brain by infusing into the bloodstream or via physical exercise or activity.
-
Lactate is mildly suppressive to the appetite, which is why immediately after exercise you're usually not hungry.
-
Having higher levels of lactate in the bloodstream lead to better outcome in patients with moderate to severe traumatic brain injury.
-
Breakdown of sugar via glycolysis in the brain is impaired in the brain after injury; however, lactate is able to bypass this process and function as fuel as a preferred energy source for brain cells.
-
When glucose is spared from being used for glycolysis it may still be available for the purpose of producing NADPH via the pentose-phosphate pathway, which is necessary for producing antioxidants such as glutathione.
-
Dr. Brooks mentions a conversation with Dr. Paul Vespa in which he characterized the "body as a thief,” and goes on to explain that it's a challenge to provide the brain with energy sources that may be used by the body before it has a chance to reach the brain.
-
Lactate transporters carry other substances, including ketones and pyruvate. This means that that the presence of too much of one substance, such as lactate, can prevent the transport of the other types. Current research is focused on developing formulations to prevent this problem of over-saturation.
-
Glucose transport in the intestinal tract is sodium mediated, which is why so many sports drinks are effective: they contain both salt and glucose, thereby increasing their effectiveness.
-
After a traumatic brain injury, receiving lactate sooner rather than later is likely more beneficial. However, in Brooks’ current research on those with traumatic brain injury, delivery of lactate usually occurs around five days after injury, due to the legal issues of treating patients in comas.
-
In the future, Brooks and his colleagues hope to gain permission to administer lactate treatment to patients immediately after brain injury, with the goal of it one day being the standard of care.
-
Traumatic brain injuries are more common in males than females.
-
Parkinson's disease patients who exercise at a level of intensity higher than they normally would perform better at motor and memory tasks.
-
Relevance for neurodegeneration Worms engineered to produce more alpha-synuclein, which, in humans, form aggregates that cause Parkinson's, had better mitochondrial function and cell viability when fed lactate.
-
Lactate is preferred over glucose even in muscle tissue when given to men during exercise.
-
A study from a group in Amsterdam showed that during exercise the brain actually has a greater energetic demand during exercise, and it meets this extra demand via lactate produced from the muscles when working out.
-
No lack of glucose for brain during traumatic brain injury, but it is not used by the neurons despite its presence. Identifying other fuels that can get into the brain, such as monocarboxylates is another line of current research.
-
Lactate present in fermented foods such as sauerkraut, kimchee, cheese, and yogurt can be taken up via lactate transporters in the gut.
Rhonda: Dr. Rhonda Patrick here. Today, I'm very excited to be sitting here with Dr. George Brooks, who is a professor in the Department of Integrative Biology at the University of California, Berkeley. He is the Director of the Exercise Physiology Lab. And he's really the pioneer of research that he proposed back in the early '80s that has to do with lactate that is generated from muscle during exercise and how that lactate is used by a variety of different tissues in the body to produce energy.
So, we'll dive into that in a few minutes. But, before we get started, I do wanna mention just a couple of things about lactate. And that is that it is something that is produced during exercise and it's used by the heart and the brain as a preferred source of energy. I'll let Dr. Brooks get into that in a little bit. And also, we're gonna talk a little bit about lactate in the context of the brain and how the brain also prefers to use lactate as a source of energy and how this is relevant for situations like traumatic brain injury, which is the focus of some of George's current research, and also for neurodegenerative diseases.
So, George, thanks for joining us. Do you mind telling us a little bit about, first of all, how you got into this research in terms of exercise, physiology, and all things lactate?
George: Yeah. Thank you, Dr. Patrick, Rhonda for coming here today so we can talk about our lactate shuttle theory and the work we've done and the trail we've followed to get to this point. So, I was an intercollegiate athlete and very much interested in running the 440 yards and the 400 meters, and I wasn't quite as good as I wanted to be. And I asked my coach, "What's the problem?" He said I had too much lactic acid, I had an oxygen debt. And so, I asked him about that, and he said, "Go read about it," and I did.
And I read about the wonderful work done by a series of investigators, going back to Nobel laureates, A. V. Hill and Otto Meyerhof. And I tried to fit that in the context of my own performance and it didn't seem to make sense. Why is lactate high when there's an energy demand? People have thought that lactate is a dead-end metabolite, that it's a poison. But, why is it always there? Why is the fire department always there at a fire? Is lactate the cause of the problem or is it there to mitigate the problem, just like the fire department?
So, if you looked at a big blaze and you saw the fire engines, you would be remiss to conclude that the fire department had started the fire. You might correctly think that it was there to mitigate the problem. And so it is in nature, when there's an energy crisis, there's a demand, the body makes lactate. Now, it can make it in excess and it can pile up and become a problem in and of itself, but we make lactate all the time.
And I don't mean to correct my lovely host here, but glycolysis, the breakdown of sugar and glycogen, happens all the time in all cells. And we use it. We use it as a fuel. We use it to support our blood sugar level. We use it as a signaling molecule to activate certain metabolic processes. It's made in some cells and it's used in other cells. And the cells which use it are cells like the heart, the liver, and we found, with the brain. And our most recent work is with neurosurgery at UCLA. We have wonderful colleagues there.
So, my partner in research, Mike Horning and I, have worked with our colleagues at UCLA and people suffering traumatic brain injury, and we think we have some really good ideas about how to mitigate the metabolic crisis that happens after injury. And we hope to be able to intervene to rescue people and improve long-term outcomes.
Rhonda: Wow, that's fantastic. So, let's step back a little bit to the lactate produced during exercise and how that...can you explain the difference between producing lactate during exercise and lactic acid and how there is this lactic acid buildup and how that may or may not cause muscle fatigue? Because that's something I think a lot of athletes are particularly concerned about.
George: Yeah. So, as I mentioned, the lactate builds up and causes an acidosis. But, when it builds up, there is a problem. The problem is the removal. So, we produce lactate and we use it all the time. And, as I mentioned, there are some really important processes that we use lactate for. So, when an athlete is going all out, lactate gets really high because the energy demand of the activity requires rapid glycolysis and glycogenolysis, and people produce lactate. But we've found, in our research, that active muscles, the heart, the liver, and the brain, are all using this stuff as a fuel. So, as long as you can make it and use it, you're fine.
Rhonda: So, is there a difference between... In order to use lactate, in order for, you know, the different organs in our body to use lactate, it needs to be taken up into the cell and then it gets into the mitochondria, and the mitochondria use this lactate as a source of energy to produce the energetic currency of the cell, ATP. Is there a difference between the type of exercise you do and or how hard you train and the ability to use this lactate, which, essentially, has to get transported into the cell through this specific lactate transporter?
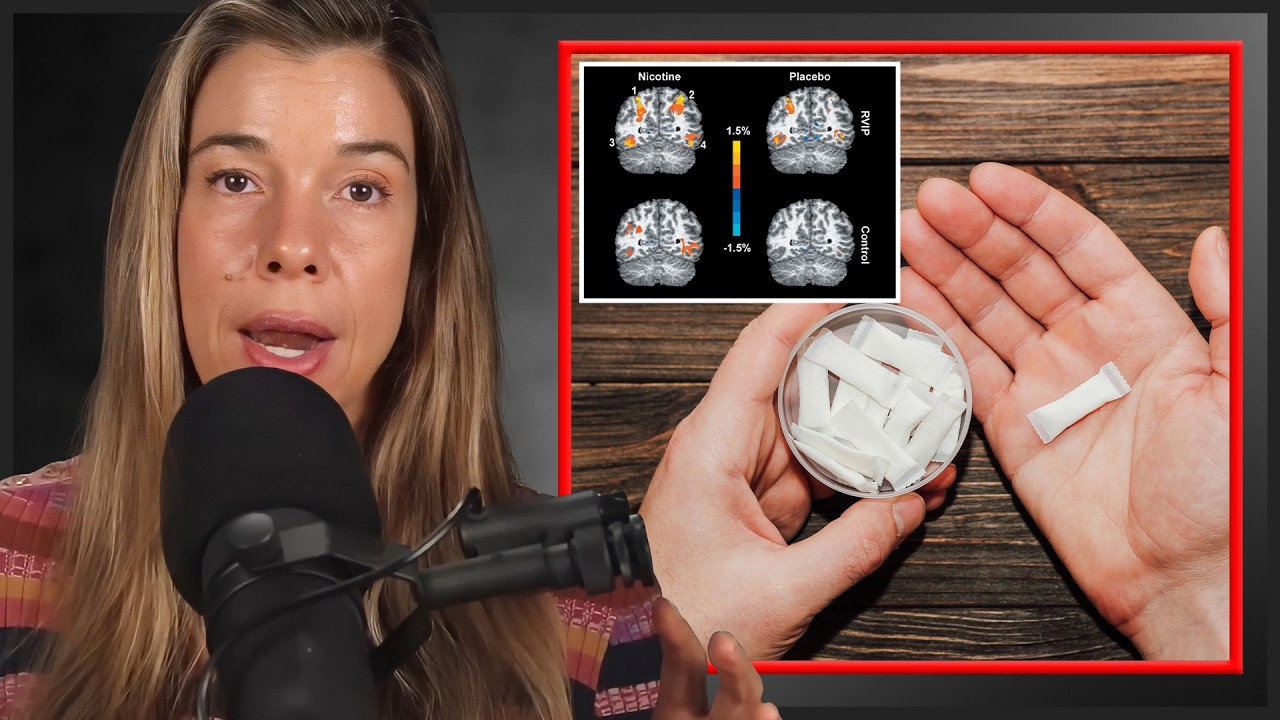
George: Yeah. So, you've mentioned some really keywords in our research. So, we've found that lactate is produced and we've found that it's oxidized. And the sites of oxidation are in the mitochondria or this network, this reticulum of a respiratory apparatus that exists in most cells. So, the lactate has to get in and it has to have a transporter. And we were amongst the first to work on discovering the lactate transporters and where they are.
And our unique contribution was to find that not only are they in the plasma membranes of muscles and heart and other tissues, but they're also in the mitochondria. They are the lactate/pyruvate transporter. So, this whole field has been misunderstood historically. And going back to the first research of Otto Meyerhof, who's one of our heroes in the study of metabolism, he had half a frog, hemicorpus, in a jar without oxygen, without perfusion, and he stimulated the muscles to contract. And as they needed energy to contract, they produced lactate. Eventually, those muscles fatigued and there was a lot of lactate.
So, this is the birth of the idea that lactate causes fatigue and that lactic acidosis is a metabolic problem. But, really, in that situation, there was no oxygen for respiration. So, the only thing the muscle could do was to break down glycogen and make lactate to try to provide energy. And, in the end, when the glycogen was used up and the lactate was high, well, there was a metabolic crisis.
Rhonda: That makes sense. So, in the context of a living person that's, you know, using oxygen and there's lots of oxygen present, like, for example, especially during exercise when we're consuming more oxygen, would you say that lactate, then, isn't gonna build up as readily and it's gonna be, instead, used to produce more energy?
George: Yeah. So, one of the things we found is that the classic training response that people have is to have a lower lactate at any power output. So, if I would compare myself to a young, healthy athlete, that athlete would actually be producing more lactate than I would be, but my lactate level would be really high because I'm limited in my ability to take it up and use it, whereas the athlete is superior in terms of clearing lactate and using it as a fuel, and using it as a gluconeogenic precursor.
So, we have discovered that lactate's produced all time, at rest and during exercise as well, and athletes actually produce more than people who are less capable or less highly trained. But, they remove it. They use it.
Rhonda: Okay. So, the athletes can produce more and they can use it better. Is that because there's something that occurs, like, during exercise that makes the transporters more efficient, makes more of them?
George: Yes. The physical activity is known to increase the mass of the mitochondrial network. Some people would say there are more mitochondria, but we know, from our own work, that this is a whole network and the mitochondrial network buds and branches out with training. So, you can actually double the amount of mitochondria you have in your muscle by training. So, that's really one of the most incredible adaptations we know about.
And, as well, you can double the amount of lactate transporters. And so, you can really upregulate the ability to do this process we call the lactate shuttle. And, again, what we mean by the lactate shuttle is some cells use it, some cells produce it, others use it and they use it well. And when the lactate is going up, that means you're not able to clear adequately.
Rhonda: Okay. So, here's the question that I have, which, I think, is probably very relevant to a lot of people out there that are listening that exercise and they're athletes, is there a certain type of exercise that is paramount in terms of doubling the mitochondrial mass, making more mitochondria, and making more of these transporters to transport lactate into the mitochondria to use it as energy? So, for example, do you...you know, aerobic exercise, like, more endurance-related running, lifting weights. Is there a certain type of exercise that you've found to be superior in that regard?
George: Yeah. We haven't, you know, really even scratched the surface of this. But, before we get too much into the science, I wanna talk about my colleagues and friends who are coaches. They have known this, sensed this metabolic crisis problem for decades and they've tried different ways to try to mitigate the problem. So, they've tried endurance training, and they've tried interval training, and they've tried combinations of endurance and interval training.
And we know, from our work on animals and people, that endurance training does increase the mitochondrial mass and does increase the number of transporters. That's all well and good. What we don't know is how to personalize it for you and me. What do we need to do, exactly, to optimize our mitochondrial mass and our lactate transporter count? What exactly? We sort of know what we need to do, which is, build the aerobic base and just do a lot of training early in the season.
Then, if we're gonna go into competition, we need to exercise harder, we need to work on our pace, and it gets really specific about our activity. But, we know, from our studies that we've done with incubated muscle cells, that if we just take muscle cells and we incubate them with lactate, it will upregulate over 600 genes, all the genes, basically, of muscle adaptation.
So, there are a lot of really talented people studying muscle adaptation today. But this has, again, been overlooked because everybody believes lactate is bad. But it's really an upstream signal which activates genes for mitochondrial biogenesis, for muscle protein synthesis, basically, pretty much what happens to you when you do exercise training.
Rhonda: Wow. So, that's fascinating George. I'm a little heavy on the biochem, so let me just recap that for those of you that may have gotten just a little lost. Basically what George is saying is that, lactate, in addition to being used as an energy source to produce energy for the cell, it also is a signaling molecule that seems to regulate maybe even on an epigenetic level where it's upregulating, meaning increasing the transcription of a variety of genes that are related to mitochondrial function and muscle adaptation and presumably, probably down-regulating genes as well.
But, essentially, it's, you know, regulating a variety of different physiological processes in the body and, I guess, in the muscle cells specifically is what you looked at, in terms of, you know, things that are good for the mitochondria and good for the muscle to work better and harder. Also, I think that the interesting thing that you mentioned about this personalized training, I think that's a very interesting concept because, you know, you're right, there's a lot of different factors.
It's not just endurance versus interval training. There is age, there is gender, you know. There is how well-trained people are. Are they trained or untrained athletes? Are they novices? How often do they train? I presume all these factors would play a role in a lot of these muscle adaptations and ability to produce more mitochondria and more lactate and transport lactate in the cell.
George: So, you know, you didn't really simplify it by talking about epigenetics, but we'll come back come back to that. But, again, our colleagues who are coaches have actually use what's called the lactate threshold as a point of training. And so, sometimes, for endurance training, people will train below the lactate threshold, just below it, and that seems to result in significant mitochondrial biogenesis.
But then, to exercise you really hard, coaches know you need to exercise above the threshold occasionally. But that's too hard to do on a daily basis. So, coaches and athletes have learned just empirically to measure lactate. They don't really completely understand what it's doing, but they know that it when it's too high, it's bad, when it's increasing, it's too hard. And if the lactate is really low, it's probably too easy training.
So, coaches and athletes are using what they call threshold training and quite effectively. And you can see, as people do their endurance training, their lactate level for a given power output really falls. So, that allows them, then, to ramp up their training and ramp up their competitive pace and then be able to function effectively at a higher power output. So, there are various things that people can do to track their training.
One, is to measure the heart rate. The other thing is to measure their lactate, and you need devices to do this. Another thing to do is to work on their breathing. Because as the lactate starts to rise, this acidosis goes on. And I often talk to people about using the talk test. So, if we were on two treadmills here when we were having this conversation, and we got going faster and faster, we would get to the point where we couldn't talk anymore. That's probably...we're acidotic now, right? So, our lactate would be really high. So, we can actually use our breathing as a measure of, you know, where we need to train.
Rhonda: That's very interesting. So, because the amount of oxygen that we're able to take in and bring, you know, to our various cells including the muscle, then, really is the limiting factor in the sense where, because if you don't have enough oxygen there, the lactate you produce isn't gonna get used. It's gonna...
George: It's gonna pile up.
Rhonda: ...instead pile up. Can you explain? Because our muscles produce lactate. And...
George: All the cells.
Rhonda: All the cells.
George: Our red blood...
Rhonda: Our lymphocytes, our...
George: Our red blood cells that we're using.
Rhonda: Right. I'm talking about it in the context of exercise because I think that's what people mostly relate it to. But, when we do produce, you know, lactate, how does it get converted into lactic acid?
George: Well, If you look the glycolytic pathway, it actually makes lactate. But, lactate is a pretty strong acid so it associates with water and a certain amount of acidosis that will be associated with the lactate itself. But, glycolysis actually makes lactate not lactic acid, the acidosis. The hydrogen ions come from other processes like the splitting of ATP, which is our high-energy energy source. And we split that, we liberate a proton. So, when you're splitting your ATP and you're not able to keep its level high, you're gonna develop protons, and the lactate is gonna be there and people are gonna associate the proton with the lactate and call it lactic acid. This is a big misnomer and big mistake in biology.
Rhonda: And at physiological pH, does the lactic acid form its lactate or?
George: The pK, okay, really is about 3. So, if there was lactic acid, it would be completely dissociated to a lactate anion proton. But, you know, one of my inventions early on was to make what I called "Polylactate" which is in the sports drink Cytomax by Cytosport in Benicia, California. And this sports drink has been used by some of the world's best athletes and it contains a lactate polymer.
And I remember from chemistry that the salt of an acid is a base. So, when we take our Polylactate and people consume it while they're exercising, their blood pH actually rises slightly.
Rhonda: Oh, interesting.
George: So, you can use the salt of an acid as a base. And so, the endogenous acid, combined with the lactate we give is removed as lactic acid, and so it actually alkalinizes the blood. And, of course, that's one of the ideas that's really, I think, one of the reasons we formed this collaboration with UCLA. Because, when people have traumatic brain injury, there is a series of things which happen. There might be an acidosis, there might be a lack of oxygen, there's swelling which goes on. And by giving salts of lactate, we can mitigate the swelling, we can provide fuel, and we can manage the acidosis which occurs.
Rhonda: Yeah. So, this is a great transition into the brain. But, before we get to that, real quick, I have one question for you. Now, you were on a committee, a scientific advisory committee, some years ago you were asked to be on, that recommends the amount of daily exercise that a person should get. And the Surgeon General of this committee, I think, recommended 30 minutes a day, but you recommended twice that. You recommended one hour a day. Can you elaborate on why that is, why we all should be exercising one hour a day?
George: Well, we didn't say everybody needs to exercise an hour a day, but this is a report we wrote when I was on the committee, and it's called "Dietary Reference Intakes." And this was the scientific basis behind what were going to become the dietary recommendations. And we turned the whole thing around. So, in the past, you would say, "What should you eat?" And you would say, "You need to eat some carbohydrates, you need some lipids, you need some fats," and so on. And then, people would recommend proportions and amounts.
We decided to first ask the question, "What are you doing? What's your energy expenditure?" And then, you need to eat to feed that. So, instead of, "Two portions of this and three portions of that," we decided to base the whole thing on energy. And what we found, by looking at the Doubly Labeled Water Database, which was generously donated by almost every investigator who ever did a study, is that people who are healthy and lean and freely living, that is eating whatever they want, they're active about an hour a day.
Now, it's true there's good epidemiological research to indicate that if you do some activity, like 30 minutes a day, you'll improve cardiovascular function, you'll reduce your risk of heart disease and diabetes and maybe some forms of cancer. But that's not enough activity to control your body weight. So, you need to be active, on the average, about an hour a day. And that's not running on a treadmill for an hour, it's doing the equivalent of brisk walking, taking the stairs, going to the bus stop, walking my students between classes, for people on their jobs, perhaps standing, moving around once in a while.
So, people who are active about an hour a day can manage their body weight and be healthier because of the activity. And the activities just, you know, are really incredible because physical activity, I say, works from the tip of your toes to the top of your head. It'll build your bone mass, it'll build your muscle mass, it'll help your cardiovascular conditioning, it will help your endocrine functioning. And actually goes up to the brain and is used as a fuel there, and it might stimulate what's called BDNF, brain-derived neurotrophic factor. So, that's another whole field we probably don't have time to talk about. But, there are things released from active muscles into the blood during activity that promote cell proliferation, like in the brain, with the BDNF, or stimulate or repress cancer cells.
So, for instance, if somebody is regularly active, why is the incidence of colon cancer reduced? Why is the incidence of breast cancer reduced? Well, these tissues are not really involved in the activity per se, but there's something released from active tissues that circulates which helps all tissues. And we think that might be lactate, but there could be cytokines, there could be micromolecules. There's probably some combination of factors released from working muscle that gets around the body, makes people feel better, and makes their tissues feel better.
And, of course, if you do enough activity, you can lose weight. But, you can be healthier without losing weight. So, depending on your day and on your schedule and what you can do, if you're active 30 minutes a day, you'll probably get some protection. But, if you are active an hour a day, you'll get a bit more protection and you can help manage your body weight.
Rhonda: Yes, I completely agree with you in terms of the BDNF, which is a neurotrophic factor, it actually stimulates the growth of new brain cells. Lactate, specifically, has been shown to stimulate BDNF, which is quite interesting because...
George: Isn't it?
Rhonda: Yeah. I mean, lactate, like you mentioned earlier, it seems to be not only the source of energy, but it seems to be a signaling molecule as well, you know. So, it's something that is good for the cells. It's good for, you know, many different cells including cells in our brain. And something else that's very interesting, very recently, I think it was a group in the Netherlands, they showed that lactate actually stimulates these neurons in a brain region called the locus coeruleus, which is where all the norepinephrine neurons are.
So, these neurons that make norepinephrine. Norepinephrine is that neurotransmitter that's involved in focus and attention. And they've shown that actually, specifically, lactate, stimulates the release of norepinephrine from those locus coeruleus neurons. And if you think about it, I know, from my own personal experience, that after I exercise, I definitely have a better focus and attention.
So, you know, this is something that seems very relevant for people with, for example, ADHD, or people that have problem focusing, you know, and putting their attention to one thing. Get out and move. Get some exercise, you know. It seems like the best medicine you can possibly have.
George: So, the terminology we use here connotes meaning, right? So, I remember being a student and learning about the blood-brain barrier and I thought there was this extra membrane that the brain was wrapped in. And that's not the case at all. Things get into the brain cells by transporters, and these lactate transporters are highly expressed in all the capillaries in our body, including in our astrocytes and our neurons.
So, a lactate permeates through the brain. When it's high, it goes in because lactate simply moves down a concentration gradient. And so a way to increase the concentration is...well, one way is, like we're trying to do with our patients, to infuse it. But, these are people who are in a coma and they can't move around. Maybe, we could try electrical stimulation to get their muscles going to fuel their brain.
But, the way we usually do it is we move around. It goes to our brain. And, you know, I don't know if you ever noticed this, after you're exercising, you get hungry, but later. Not right away. Because the lactate is crossing the blood-brain barrier. It's probably going to the centers in the brain that regulate appetite and it's mildly suppressive.
Rhonda: Interesting.
George: And so, but then, when the lactate clears, is used, you know, by the body, it uses it, then you get hungry.
Rhonda: The brain says, "Feed me."
George: "Feed me."
Rhonda: So, this concept of lactate helping with traumatic brain injury, you mentioned you're doing a current research collaboration with researchers at UCLA. And I read, you know, a couple of papers recently on this topic of lactate in terms of patients that had moderate-to-severe traumatic brain injury and they had higher levels of lactate in their bloodstream, they had a better outcome. And those patients that, you know, did not have an intact blood-brain barrier, that that wasn't the same for them. So, can you explain why lactate would help people recover better from traumatic brain injury?
George: Sure. Can I tell you a story first? Just in this office, almost eight years ago, the Chief of Neurosurgery at UCLA, Dr. Neil Martin and one of his chief scientists, Dr. Tom Glenn, we had a conversation in this office about this. And they noticed that in their patients who did better, as you described, their lactate was elevated and the people whose brains could take it up did better. And that means that they recovered quicker and their intellectual functions were restored pretty much as they were before they were injured.
And they wrote a grant, and the grant went to the NIH. And they said, "Well, you just don't understand." So, they looked in the literature and they came to us because we'd been dealing with this issue for decades. People just think we're crazy because this stuff obviously is bad, it can't be helpful. So, we started working with patients and we have recently written two papers. We call one paper, "The Body Paper," and the other paper, "The Brain Paper."
And now, we can see that, after injury, the whole body knows the brain is injured and the whole body mobilizes its efforts to fuel the brain. And part of that is lactate being produced peripherally. And it does a couple things. Of course, it goes to the brain and it can get across this blood-brain barrier and can help fuel the brain. The other thing it does normally is the lactate goes, of course, to the liver and it gets made into glucose, and the glucose is usually used as a fuel for the brain.
Now, to answer your question about why is the injured brain suffering. For some reason, the breakdown of sugar - glycolysis - in the brain is impaired after injury. The product of that is lactate. And neurons run on lactate as the preferred fuel. So, in part, the brain is starving. So, you can try to give more glucose by putting that in the blood. But, again, the process is blocked.
So, our approach is to bypass that by infusing formulations containing lactate salts and esters other lactate-containing compounds that will get into the brain through the blood-brain barrier, and get into the mitochondria, and fuel the brain. And we actually have done six patients. In collaboration with our colleagues at UCLA, we show that we can increase the carbohydrate uptake, that is the total of glucose plus lactate, in people with brain injury by infusing lactate.
Rhonda: Wow. Do you think that some of the glucose that is in the brain instead of being used for an energy source is actually being used to produce things like NADPH? So, it's going through this different metabolic pathway called the pentose phosphate pathway.
George: Yes.
Rhonda: So that you can make things that are, essentially, gonna be used as antioxidants by things like glutathione peroxidase and these other antioxidant enzymes that are in the brain. Do you think that lactate will then allow glucose to be used for that instead of as energy for neurons?
George: Yeah. So, you're talking about sparing.
Rhonda: Yes.
George: You're talking about glucose sparing. And so, you described, very nicely, the pentose phosphate pathway and some of the things it does. So, there is a limitation in the amount of glucose, but if we can fuel the brain with lactate, then the glucose that is available can do that as well as help fuel the brain. So...
Rhonda: Which is very important because...
George: It's very important. You know, in our approach as physiologists to this issue, we talk about the body supporting the brain. Too often, in the past, people get into silos. So, if I'm a muscle physiologist, I don't care about the brain. And if I'm a neurosurgeon, I really wanna feed the brain because that's where my patients are and that's what I have to do.
But, we have to just step back and say, "Hey, the brain is in the body. We have to nourish both. We have to realize that the body is gonna nourish the brain." And in one of our conversations I had with one of our colleagues was with Dr. Paul Vespa, who is a neurologist who really takes care of the patients after their surgery is over. "Dr. Vespa," I said, you know, "The body is a thief," and he looked at me and he got it right away. Because, a person who's in the intensive care unit and they're being treated by standard of care and the best physicians in the most highly advanced medical centers are concerned with bleeding and oxygenation. And it's really hard to nourish people in these circumstances, to feed them. They're unconscious, they can't eat. You have to put in tubes, you have to put in things that go in the blood, things that go in the nasogastric tube into the stomach.
Rhonda: Repairing things.
George: Yeah, you need to have fuel, you need to have nutrients. And so, it's hard to get this aboard. So, Dr. Vespa's idea was, "I'm gonna inject something into the blood and it's gonna go to the brain and help the person." Yes and no. Because the body's gonna grab it too. So, then, we have to find a way to nourish the whole organism, the body and the brain.
We think we've done that. And we have some patents pending about how to, with a minimum of invasiveness, find out what the body energy state is and to make formulations that will nourish the body and the brain so the body won't be stealing from the brain instead of reinforcing the brain. So, we have this notion of a holistic approach of body and brain. And the body supports the brain, that's true. And we need to accommodate both.
Rhonda: Exactly. So, this leads me to the supplementation with lactate. There is L-lactate and there is D-lactate. Is there a difference between supplementing with L-lactate versus D-lactate?
George: Well, I can, with my hands here, for the camera, show you guys the racemic nature. We have an L-lactate, I'll use my left hand, and this is one configuration. The mirror image is D-lactate. And D-lactate is actually neurotoxic. Okay. So, in the past, there was a solution, or is still used prominently called lactated Ringer's solution. And Ringer was a 19th century physiologist, pharmacologist, maybe physician, who developed a formulation so cells could live in vitro, in a dish, and formulations to give to a person to support them after injury. And so, what was available was lactate, and he tried it and it seemed to work.
But, at that time, it was a 50-50 mixture. So, we can improve standard of care by taking out the D and just using L. And we can find different things to carry this molecule. So, one of the things that I have invented in the past was arginine lactate to use a basic amino acid with a positive charge to bind a negative charge. And we used that in Cytomax, but we could also give it to a person after injury.
And, of course, sodium is the major one. And sodium is good because sodium supplementation to somebody with injury would help mitigate swelling of the injured tissue. So, there are different vehicles and different ways to deliver these formulations. I wanted to talk about formulations. We realized that lactate transporters also transport ketones and pyruvate as well as lactate.
So, we can come up with formulations and mixtures of these things which are transportable and can be used. And in our conversation we had with Dr. Ames just the other day, you know about the ketotic diets and the management of conditions in pediatrics. So, they come also in by these same transporters. And the thing about it, and once you realize that there are transporters and understand how they perform, they're saturable. So, if we have, then, all lactate, then that'll probably block ketones, and if we have all ketones, that'll block lactate.
So, we don't know yet which percentage of what will work the best. Right now, since the MCTs, the monocarboxylate transporters, are more specific to lactate than pyruvate, than other monocarboxylates, our presumption is to give lactate compounds intravenously. But, we also know that if somebody is recovering maybe if they're on a ketotic diet, the ketones will help supplement what we deliver intravenously. We'll have to find out.
Rhonda: Wow. So, you brought up some really important points here. But, first, I just wanna ask you then, with the D and L-lactate, the D-lactate is neurotoxic. Does it block lactate from being transported into the cell? Is it because they're essentially mirror images of each other? What is so toxic about the D?
George: Yeah. I'm not sure about why it was toxic, but, no, they don't share the transporter. So, actually, one of the tests we did early on, we showed saturation kinetics with L-lactate. And D comes in a linear fashion, so it probably comes in simply by diffusion. So, fortunately, it doesn't share the transporter, which is probably why it's not so neurotoxic in vivo. Because it's hard for it to get in. It gets disposed of, probably by the liver, whereas the L can get into the brain.
Rhonda: Because I know that certain, for example, you know, yogurt, they've got these little bugs in there that make all sorts of, you know, lactate. And some of them are L, some of them are D. I think majority of them are L, but you do have some bacterial strains that make D. So, you know, that's something to keep in mind, is looking at, you know, what probiotics are a big thing now also. So, you're getting probiotics that make D-lactate may not be as good as getting probiotics that make L-lactate.
George: I would avoid them.
Rhonda: You would avoid the D-lactate?
George: Yeah. So, you know, with Cytomax, I realized early on that there was an intestinal transporter, a lactate transporter. So, a different gene family than the one that's in the cell membranes. And what's neat about it is it's sodium-mediated. It's not a symport for protons, it's a symport for sodium. So, of course, we put a little sodium lactate in our sports drink and that helps to facilitate lactate uptake in the GI tract.
And also, the glucose transporter in the intestinal tract is a sodium-mediated one. So, you know, that's how these sports drinks really work very well and work better than water, regardless of the brand. Because if you have glucose and a pinch of salt, it's gonna get in faster than glucose alone.
Rhonda: Interesting. Very interesting. Okay, sodium lactate, good for the gut, good for the body.
George: Yeah. It's taken up much faster than glucose. So, there was a study done some years ago by Dr. Jack Azevedo at Chico State University. And he did this study where he isotopically labeled the individual components of the favorite brand, the best known brand, and also Cytomax. Cytomax contains three kinds of carbohydrates, a lactate, a glucose, and a pyruvate. A lactate, and a glucose, and a fructose transporter. So, three, whereas, most sports drinks have high-fructose corn syrup, which means a lot of fructose, and probably too much fructose and not enough glucose.
So, in separate trials, he isotopically labeled the glucose and fructose in the number one brand, and then the lactate, the fructose, and the glucose in Cytomax. I mean, every subject had to do five trials. And you can see the lactate is the first taken up by the intestinal tract, and it's burned and it's in the breath in five minutes. But, it takes at least a half hour for glucose to get there, reach a peak. So, if you want quick energy, we feed lactate. You can even drink it or put it in through a nasogastric tube or put it in the blood. Because of all these transporters and their abundance, they get in right away and they're used right away.
Rhonda: Very, very cool. You mentioned some of the ketone bodies also. So, ketone bodies are generated through beta-oxidation. And, you know, something specifically also, medium-chain fatty acids can produce ketone bodies like beta-hydroxybutyrate, which have also been shown to help with traumatic brain injury as well.
George: Ketotic diet, yeah, as a treatment.
Rhonda: Yes. So...
George: Especially in kids.
Rhonda: The question is, do you think there is a competition between lactate and these ketone bodies like beta-hydroxybutyrate, for example, in getting transported into different cells including the brain? Do you know if...so, the lactate wins, lactate can outcompete?
George: Lactate will outcompete it. So, the first studies we did in 1990, where we did isolated sarcolemmal vesicles. So, imagine a muscle that's got a big membrane sheath and you can actually take the sheath off and it'll form vesicles. And then, you can study the uptake of different things in these vesicles. And so that's how I would describe the lactate transporter. So we compared it to glucose and amino acids and to ketones. And so, lactate is preferred to get in, right? It outcompetes because it really fits the transporter configuration better than the other things do.
Rhonda: Yeah. This...
George: But, you know, in vivo, how can you deliver this stuff? Okay. As I mentioned, you can put the lactate in orally or you can put it in an IV. But, in terms of a person's nutrition ongoing, that gets kinda complicated with somebody leaving a hospital. So, maybe, a ketotic diet would really work as a recommended diet for them leaving.
Rhonda: So, what about the time frame after a traumatic brain injury? Is there a certain window that delivering this lactate infusion is gonna be the most beneficial in terms of preventing the brain from having more damage? And, you know, like I said, you're sparing glucose, you're making more antioxidants that can be used to prevent more reactive oxygen species from further damaging your brain cells and, you know, creating this whole vicious cycle.
George: Yeah. I wish I knew the answer to that. I think it's the sooner the better. My wife, Rosemary, is a physician and she deals in the area of sports medicine and physical activity. And since concussive injuries are so frequent especially in kids, not just pro athletes but kids playing sports of all ages, she really wants me to develop something that she can give to her patients, right? To give on the spot.
Now, our patients in the intensive care unit are studied about five days after injury. And the reason for that is we have to get permission from their legal representatives to do these procedures.
Rhonda: Is that because they're so experimental?
George: Well, yeah. For somebody to be in the experiment, they have to give consent. And if they're in a coma, it's their legal representative. So, we have to find these people and they have to give consent. And, you know, if somebody's in your family, just imagine somebody's had an injury and they're in a hospital, and then...it's gonna take a few days for everybody's head to clear and to give permission to make these measurements.
So, I personally think that if we instituted a lactate infusion when the person is admitted to the intensive care unit, that would probably be more helpful. But, certainly, we can see that even five days out, if we give lactate supplementation, the patients are gonna take it up and use it.
Rhonda: So, it still helps regardless of the five days later or not.
George: Yeah. So, right now, what we probably have to do is work with the institutional review boards and try to get permission to do these right-away trials. And, again, the person has a right to know that they're in an experiment, and if they're not capable of knowing that, then their family or their legal representatives have to give permission.
Rhonda: Do you think in the future, maybe, if this can become more of a standard procedure that, maybe, you know, the medics will have it on hand when they get to the...for example, let's say we have a car accident, and the car accident is the source of the brain injury, that they immediately are, you know, infusing them intravenously with some sodium lactate?
George: So right now, we have in the NIH grant proposed that we're gonna do it with animals first and see the early versus late intervention. And then, we'll have the animals and they'll be given an injury, and then we'll try to infuse them right away or later. And then, if that works out the way we think it will, then maybe we'll get consent to do this. We'll get permission to have people pre-consented to do this procedure. And then, it might become standard of care. And then, once it's standard of care, then it could be given widely.
Rhonda: That's awesome.
George: And it would be in somebody's medical kit. Think about car accidents, bike accidents, kids playing soccer, football, kids playing basketball and falling and bumping their head, this happens hundreds of thousands of times. And then, not to mention, you know, what's happening in the military with concussive injuries. And so, there's two classes of people we've been fortunate to work with. One are young men, more than women. Men drive their motorcycles and cars too fast and...
Rhonda: They also play football.
George: They play football. But, you know, women play basketball as well and do get concussed, get bumped in the head playing soccer sometimes, you know, not by the ball but by the head of an opposing player or a teammate even. So, people suffer concussive injuries. And to have something on hand to mitigate this metabolic crisis which develops after the injury would be really advantageous.
So, while there are a lot of professional football players and they need to be protected and need to be treated well, there are thousands more kids and people who suffer these injuries. So, in addition to young men, we have older people. Older people who, know, lose their balance and fall, and they might break their hip or they might get a concussion, or they might get both.
So then, it was sort of a bimodal distribution of traumatic brain injury. You know, the young men and then people of both genders later in life. So, it would be important to help everybody.
Rhonda: Yeah. So, you mentioned elderly people. And that brings to mind neurodegenerative diseases because I think traumatic brain injury is, like neurodegenerative disease, in real-time, exponential, you know. Because, brain aging also has a lot of these factors that traumatic brain injury has, reactive oxygen species, these neuroinflammatory molecules and neuroinflammation which causes accumulation of amyloid-beta plaques outside neurons, and all these things. But, that happens a little bit, you know, each day over many, many years.
So, the possible use of lactate in helping treat neurodegenerative diseases also may be an interesting point. I know that there is a couple of studies that I've been interested in. One has to do with exercise and Parkinson's disease. They've shown that Parkinson's patients that are forced to exercise beyond what they usually would, to the point where it's uncomfortable, that they perform better on certain motor tasks and also memory tasks.
And, of course, I'm thinking immediately, well, of course, you're producing lactate. Lactate is going in the brain and helping fuel some of these, you know, neurons in the substantia nigra which are dying, these dopaminergic neurons which are dying. And, just recently, like a few months ago, actually, a study came out in worms and also mice, Parkinson's model. Worms don't actually have a brain but they do have Parkinson's models for worms, where they have alpha-synuclein, which is one of the toxic proteins that aggregates in Parkinson's disease, they have these worms making that at an accelerated rate. But, they showed that feeding the worms lactate helped improve the mitochondrial function, it increased the cell viability, and also in mice.
So, you know, I think that this is the next step is, "Well, okay. Can lactate be used in the context of neurodegeneration to help the brain have that energy that it needs so that it can, you know, perform memory functions?" And also, so that it can grow new brain cells. We're talking about lactate being a signaling molecule, you know, increasing BDNF.
George: Well, you know, Dr. Patrick, you know so much about this. And you have a good colleague, CHORI, Mark Shigenaga, who you probably work with, you know. He's been working in an animal model. He's been supplementing lactate showing positive benefits. So, maybe we've just scratched the surface. Maybe, we've got the whole picture wrong from the beginning.
And so, thinking about fueling, supporting tissue function, cell signaling, these are all things that need to be explored. And there are many maladies, both chronic and acute, that understanding the basic biochemistry would really help us resolve what the issues are. And it would be, of course, a huge mistake to say this works for everything all the time. But, knowing this will be helpful in many such circumstances which we probably can't predict.
So, Parkinson's, maybe, that's one. I'm not an expert in that, I really don't know that. I'll take your word for it, I haven't read those papers. But, you know, we've done a lot of work and we can see that lactate gets into the brain, and when you exercise, it goes up in the blood and it's available to fuel, and it's preferred. And we use that word "preferred." That's very interesting because we've given lactate to men during exercise and it is preferred over glucose.
Rhonda: In what tissues?
George: In muscle and in heart.
Rhonda: In heart.
George: Yeah. And also in brain. But, here's the thing about traumatic brain injury, if lactate substitutes for glucose, that doesn't give the brain any advantage. But, after a traumatic brain injury, when the ability to use glucose is blocked, then we can supplement by giving lactate. So, if we had somebody who is just normal healthy and we gave lactate, they would spare the glucose, okay? But, here, after TBI, glucose metabolism is impaired. So, we seek to augment total carbohydrate uptake by giving lactate.
Rhonda: It's impaired in neurons or in astrocytes?
George: Well, we don't know that.
Rhonda: We don't know? Because, I always think about lactate, as a source of energy, being thermodynamically favorable because you don't need energy to convert lactate into pyruvate, but you need energy to convert glucose into pyruvate. It costs energy to make energy. So, I always think about, you know, and using lactate as a source of energy to produce more energy is thermodynamically favorable. It makes sense.
George: Yeah, it is. It's more reduced, so it actually has more energy than pyruvate. And, as you said, energy comes free. So, in brain metabolism, there's a big discussion about the astrocyte-neuron lactate shuttle. And it's been proposed that astrocytes, which are far more numerous than neurons, take glucose and make lactate, and they nourish the neurons that way.
So, if there's a shutdown in glycolysis in astrocytes, that would starve neurons. But, Raja Hussein, who just poked her head in here, she has a paper where she showed that all the apparatus for neurons to take glucose and make lactate are intact always. So, it might be a population thing. It might be the fact that there's so many astrocytes. So, they're gonna take their glucose and they're gonna make the lactate. And the neurons are gonna run on lactate because the glucose has been basically swiped by astrocytes and is not really available to the neurons.
But, in terms of this word "preferential," if lactate substitutes for glucose in somebody with a traumatic brain injury, we haven't gained anything. Right But, if glycolysis is blocked and we can augment the total energy supply by giving lactate, that's what we seek to do. Now, we know if we give lactate to an exercising person, that it will spare glucose. And that helps the person exercise.
Rhonda: Yeah, recently, earlier this year, actually, a group from Amsterdam published a study showing that during physical activity, the brain also ramps up and works harder just like the muscle cells do. And it's working harder being fueled from lactate, you know. So, you know, your brain's working harder when you're working out and lactate is allowing it to do that.
George: Yeah. And a group from Copenhagen showed that when you exercise and your blood lactate rises, it substitutes for glucose in the brain. So, that's perfectly predictable, according to lactate shuttle theory. But, again, with the injured brain where there's a limited glucose, not availability, because the doctors are very good at supplying glucose and giving insulin, although the brain doesn't respond to insulin like other tissues do, they're very good at maintaining blood sugar level. So, it's available. There's no lack of glucose for the brain. It just doesn't get in.
Rhonda: It's just not working.
George: It's just not working. So, okay.
Rhonda: So, find another way. Lactates seems to be that way.
George: So, yeah. So, yeah, monocarboxylates are another way, and lactate is the major one. People are experimenting with pyruvate. And pyruvate's not a bad metabolite to give IV, although, earlier on, it was recognized that pyruvate degrades in solution, makes toxic products. So, others have tried to keep a crystalline pyruvate on hand and mix it up in saline right away, and then give that in high levels.
But, the body so prefers lactate when you infuse it and one circulatory passage is all lactate. So, the lungs do that and red blood cells do that. The red blood cells in the lungs are loaded with enzymes to convert pyruvate to lactate. So, even if you wanna try to give pyruvate, within seconds or a minute, it's gonna be lactate anyhow, which is fine. It's the way nature's decided to do it.
Rhonda: Yeah. So, do you think that supplementing with L-lactate is something that would be beneficial to people? That don't have traumatic brain injury. Just, people like me, for example.
George: Well, you mentioned yogurt, you eat yogurt?
Rhonda: Yeah, I do.
George: And depending on ethnicity and where people are, people eat sauerkraut, they eat kimchi, they eat cheese. These are all fermentation products that have lactate in them. And, as we mentioned, there is lactate transporters in the GI tract, and it's biologically accessible. But, you know, these things are made through fermentation, they can be kinda acidic.
So, the new yogurts are different than the ones I remember when I was a kid. And they're not so sour because we add sugar and we, basically, counteract what goes on naturally in these products. So, yes. But, you know, putting in a lot of calories in terms of lactate compounds is pharmacologically viable. It'll be expensive compared to a regular food. And when you eat carbohydrates, you make lactate anyhow.
So, in terms of its availability, you know, most foods will make lactate, and the body will make it and dispose of it. And nobody actually realized this because unless you use isotope tracers like we did, you know, you eat carbohydrate and your blood lactate rises a little bit. But, actually, the production went way up. It just wasn't removed.
Rhonda: By the heart, by the liver, by the brain, by all these different tissues that are using it as a source of energy?
George: Yeah.
Rhonda: Well, this has been so enlightening, George. Thank you so much for joining us today. I really, really learned a lot about lactate and all the different physiological roles of it, in terms of the whole body, including the brain. And if people are interested in reading, you know, about your research or things that you're doing and you also mentioned your Cytomax sports drink, where can they find you?
George: Well, I've been at UC Berkeley for 43 years. So, [email protected] or [email protected], people can find me.
Rhonda: MD Flux?
George: MD Flux. So, Mike Horning and I have tried to take this knowledge. We were encouraged by a course we took at UCSF last year called "Lean Launchpad," and it was a course about how to take ideas from science to make them more widely available by developing a business. And our business is called MD Flux.
Rhonda: So, mdflux.com...
George: .com
Rhonda: People can find what's going on mdflux.com.
George: Yeah, people can find out what we're doing. And so, we've written this body paper I told you about and the brain paper, and we have a review which is being considered for publication. So, PubMed, everybody should know about PubMed, it's instituted by the Library of Congress and what's published in biology and medicine is available on PubMed. And yeah, thank you for coming and letting us talk about the lactate shuttle, which started on the track and has winded up in the brain.
Rhonda: Excellent. Thank you so much, George, for joining us.
George: You're welcome.
An energy-carrying molecule present in all cells. ATP fuels cellular processes, including biosynthetic reactions, motility, and cell division by transferring one or more of its phosphate groups to another molecule (a process called phosphorylation).
A protein present in the human brain, found primarily at the synapses – the junctions between neighboring neurons where the exchange of electrical signals and neuronal communication occurs. Aggregation, or clumping, of alpha-synuclein proteins is a hallmark of Parkinson's disease, a neurodegenerative disorder of the central nervous system. Hsp70, a heat shock protein, has been shown to reduce formation of alpha-synuclein oligomers and reduce associated toxicity.[1]
- ^ Hashimoto, Tadafumi; J. McLean, Pamela; Danzer, Karin M.; Ruf, Wolfgang P.; Putcha, Preeti; Joyner, Daniel, et al. (2010). Heat‐shock Protein 70 Modulates Toxic Extracellular Α‐Synuclein Oligomers And Rescues Trans‐Synaptic Toxicity The FASEB Journal 25, 1.
A toxic 42 amino acid peptide that aggregates and forms plaques in the brain with age. Amyloid-beta is associated with Alzheimer's disease, a progressive neurodegenerative disease that can occur in middle or old age and is the most common cause of dementia. Heat shock proteins have been shown to inhibit the early aggregation of amyloid beta 42 and reduce amyloid beta plaque toxicity [1].
A molecule that inhibits oxidative damage to DNA, proteins, and lipids in cells. Oxidative damage plays a role in the aging process, cancer, and neurodegeneration. Many vitamins and plant-based compounds are antioxidants.
Star-shaped cells found in the brain and spinal cord. Astrocytes facilitate neurotransmission, provide nutrients to neurons, maintain neuronal ion balance, and support the blood-brain barrier. Astrocytes also play a role in the repair and scarring process of the brain and spinal cord following traumatic injuries.
A chemical produced in the liver via the breakdown of fatty acids. Beta-hydroxybutyrate is a type of ketone body. It can be used to produce energy inside the mitochondria and acts as a signaling molecule that alters gene expression by inhibiting a class of enzymes known as histone deacetylases.
The process by which fatty acid molecules are broken down. Beta-oxidation occurs in the mitochondria and produces acetyl-CoA, FADH2, NADH, and H+. Under conditions where glucose is limited, beta-oxidation is an important preceding step for producing the acetyl-CoA needed for ketogenesis.
A highly selective semi-permeable barrier in the brain made up of endothelial cells connected by tight junctions. The blood-brain barrier separates the circulating blood from the brain's extracellular fluid in the central nervous system. Whereas water, lipid-soluble molecules, and some gases can pass through the blood-brain barrier via passive diffusion, molecules such as glucose and amino acids that are crucial to neural function enter via selective transport. The barrier prevents the entry of lipophilic substances that may be neurotoxic via an active transport mechanism.
A type of protein that acts on neurons in the central and peripheral nervous systems. BDNF is a type of neurotrophin – or growth factor – that controls and promotes the growth of new neurons. It is active in the hippocampus, cortex, cerebellum, and basal forebrain – areas involved in learning, long term memory, and executive function. Rodent studies suggest that lactate, one of many so-called exerkines, mediates some of the benefits of exercise on learning and memory via inducing neuronal BDNF expression.[1] Exercise in combination with heat stress increases BDNF more effectively than exercise alone.[2] BDNF is a profoundly universal point of convergence for mechanistically explaining essentially all known activities that promote brain health.
- ^ Helge, Jørn Wulff; Moritz, Thomas; Morville, Thomas; Clemmensen, Christoffer; Dela, Flemming (2020). Plasma Metabolome Profiling Of Resistance Exercise And Endurance Exercise In Humans Cell Reports 33, 13.
- ^ Heyman, Elsa; Goekint, Maaike; Roelands, Bart; Njemini, Rose; Meeusen, Romain (2011). Influence Of Citalopram And Environmental Temperature On Exercise-Induced Changes In BDNF Neuroscience Letters 494, 2.
A large class of diseases that involve the heart or blood vessels, including stroke, hypertension, thrombosis, heart failure, atherosclerosis, and more. Cardiovascular disease is often caused by lifestyle factors. As such, up to 90 percent of cardiovascular disease may be preventable.[1]
A broad category of small proteins (~5-20 kDa) that are important in cell signaling. Cytokines are short-lived proteins that are released by cells to regulate the function of other cells. Sources of cytokines include macrophages, B lymphocytes, mast cells, endothelial cells, fibroblasts, and various stromal cells. Types of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factor.
A neurotransmitter best known for its role in motor, motivation, and pleasure control. Dopamine also functions as a paracrine (cell-to-cell) hormone in other parts of the body. It is derived from tyrosine and is the precursor to norepinephrine and epinephrine. Some evidence suggests that dopamine may also be involved in pain modulation.
Any of a group of complex proteins or conjugated proteins that are produced by living cells and act as catalyst in specific biochemical reactions.
Genetic control elicited by factors other than modification of the genetic code found in the sequence of DNA. Epigenetic changes determine which genes are being expressed, which in turn may influence disease risk. Some epigenetic changes are heritable.
A molecule composed of carboxylic acid with a long hydrocarbon chain that is either saturated or unsaturated. Fatty acids are important components of cell membranes and are key sources of fuel because they yield large quantities of ATP when metabolized. Most cells can use either glucose or fatty acids for this purpose.
The process in which information stored in DNA is converted into instructions for making proteins or other molecules. Gene expression is highly regulated. It allows a cell to respond to factors in its environment and involves two processes: transcription and translation. Gene expression can be turned on or off, or it can simply be increased or decreased.
A survival mechanism the brain relies on during starvation. Glucose sparing occurs when the body utilizes fatty acids as its primary fuel and produces ketone bodies. The ketone bodies cross the blood-brain barrier and are used instead of glucose, thereby “sparing” glucose for use in other metabolic pathways, such as the pentose-phosphate pathway, which produces NADPH. NADPH is essential for the production of glutathione, one of the major antioxidants used in the body and brain.
An antioxidant compound produced by the body’s cells. Glutathione helps prevent damage from oxidative stress caused by the production of reactive oxygen species.
An antioxidant produced within cells that enzymatically reduces hydrogen peroxide to water to limit its harmful effects. Glutathione peroxidase's primary role is to protect cells from oxidative damage, a key factor in many diseases.
A highly branched chain of glucose molecules that serves as a reserve energy form in mammals. Glycogen is stored primarily in the liver and muscles, with smaller amounts stored in the kidneys, brain, and white blood cells. The amount stored is influenced by factors such as physical training, basal metabolic rate (BMR), and eating habits.
A series of enzyme-dependent reactions that breaks down glucose. Glycolysis converts glucose into pyruvate, releasing energy and producing ATP and NADH. In humans, glycolysis occurs in the cytosol and does not require oxygen.
A critical element of the body’s immune response. Inflammation occurs when the body is exposed to harmful stimuli, such as pathogens, damaged cells, or irritants. It is a protective response that involves immune cells, cell-signaling proteins, and pro-inflammatory factors. Acute inflammation occurs after minor injuries or infections and is characterized by local redness, swelling, or fever. Chronic inflammation occurs on the cellular level in response to toxins or other stressors and is often “invisible.” It plays a key role in the development of many chronic diseases, including cancer, cardiovascular disease, and diabetes.
A peptide hormone secreted by the beta cells of the pancreatic islets cells. Insulin maintains normal blood glucose levels by facilitating the uptake of glucose into cells; regulating carbohydrate, lipid, and protein metabolism; and promoting cell division and growth. Insulin resistance, a characteristic of type 2 diabetes, is a condition in which normal insulin levels do not produce a biological response, which can lead to high blood glucose levels.
Experiments that are performed using cells or microorganisms outside of their normal biological context and are often done in a test tube or petri dish.
A diet that causes the body to oxidize fat to produce ketones for energy. A ketogenic diet is low in carbohydrates and high in proteins and fats. For many years, the ketogenic diet has been used in the clinical setting to reduce seizures in children. It is currently being investigated for the treatment of traumatic brain injury, Alzheimer's disease, weight loss, and cancer.
Molecules (often simply called “ketones”) produced by the liver during the breakdown of fatty acids. Ketone production occurs during periods of low food intake (fasting), carbohydrate restrictive diets, starvation, or prolonged intense exercise. There are three types of ketone bodies: acetoacetate, beta-hydroxybutyrate, and acetone. Ketone bodies are readily used as energy by a diverse array of cell types, including neurons.
Lactate is thought to participate in a sort of "lactate shuttle" where, after being produced in muscle from exercise, it is transported in to tissues like the heart, and brain, where it is used as an energy source. Lactate is one of many molecules that falls under a loose group of molecules referred to as exerkines, a broad group of exercise-induced hormonal-like factors. Evidence suggests that lactate is the preferred fuel of the brain. Additionally, rodent studies suggest that lactate mediates some of the benefits of exercise on learning and memory via inducing neuronal brain-derived neurotrophic factor (BDNF) expression.[1] In clinical studies, lactate shows promise as a treatment for inflammatory conditions including traumatic brain injury and as a means to deliver fuel to working muscles.
- ^ Helge, Jørn Wulff; Moritz, Thomas; Morville, Thomas; Clemmensen, Christoffer; Dela, Flemming (2020). Plasma Metabolome Profiling Of Resistance Exercise And Endurance Exercise In Humans Cell Reports 33, 13.
Lactate that is produced from an oxygen-independent metabolic pathway (glycolysis) is shuttled to various tissues including muscle, heart, and brain, where it is used as a substrate for oxygen-dependent energy production.
A medical condition characterized by the buildup of lactate in the body and can occur as the result of an underlying acute or chronic medical condition, medication or poisoning.
The thousands of biochemical processes that run all of the various cellular processes that produce energy. Since energy generation is so fundamental to all other processes, in some cases the word metabolism may refer more broadly to the sum of all chemical reactions in the cell.
Tiny organelles inside cells that produce energy in the presence of oxygen. Mitochondria are referred to as the "powerhouses of the cell" because of their role in the production of ATP (adenosine triphosphate). Mitochondria are continuously undergoing a process of self-renewal known as mitophagy in order to repair damage that occurs during their energy-generating activities.
The process by which new mitochondria are made inside cells. Many factors can activate mitochondrial biogenesis including exercise, cold shock, heat shock, fasting, and ketones. Mitochondrial biogenesis is regulated by the transcription factor peroxisome proliferator-activated receptor gamma coactivator 1-alpha, or PGC-1α.
A substance produced in the brain. Norepinephrine acts as a hormone and neurotransmitter and is best known for its role in the body’s “fight or flight” response to stress. Its role as a neurotransmitter has been exploited as a molecular target for a class of drugs known as norepinephrine reuptake inhibitors, which were developed for the purpose of treating disorders ranging from ADHD to narcolepsy and depression. Norepinephrine also plays a role in converting white adipose tissue into brown adipose tissue via an uncoupling protein 1 (UCP-1) mediated mechanism.
A neurodegenerative disorder that affects the central nervous system. Parkinson’s disease is caused by destruction of nerve cells in the part of the brain called the substantia nigra. It typically manifests later in life and is characterized by tremors and a shuffling gait.
An alternate pathway for the oxidation of glucose. The pentose phosphate pathway parallels glycolysis, but does not require or produce ATP; rather, it produces NADPH, which is necessary to create the cellular antioxidant glutathione. Like glycolysis, the pentose phosphate pathway occurs in the cytoplasm.
pK is a measure of proton binding affinity. If a compound or molecule has low pK (less than 2.5), it has low affinity for binding protons. Strong acids have low pK. If a compound or molecule has high pK (greater than 3.0), it has high affinity for binding protons. Weak acids have high pK.
One of the enzymes involved in the process of converting pyruvate, which is derived from glucose, into energy in the form of ATP inside of the mitochondria.
Oxygen-containing chemically-reactive molecules generated by oxidative phosphorylation and immune activation. ROS can damage cellular components, including lipids, proteins, mitochondria, and DNA. Examples of ROS include: peroxides, superoxide, hydroxyl radical, and singlet oxygen.
A related byproduct, reactive nitrogen species, is also produced naturally by the immune system. Examples of RNS include nitric oxide, peroxynitrite, and nitrogen dioxide.
The two species are often collectively referred to as ROS/RNS. Preventing and efficiently repairing damage from ROS (oxidative stress) and RNS (nitrosative stress) are among the key challenges our cells face in their fight against diseases of aging, including cancer.
A molecule that allows cells to perceive and correctly respond to their microenvironment, which enables normal cellular function, tissue repair, immunity, cognition, and more. Hormones and neurotransmitters are examples of signaling molecules. There are many types of signaling molecules, however, including cAMP, nitric oxide, estrogen, norepinephrine, and even reactive oxygen species (ROS).
A compound, CO(NH2)2, occurring in urine and other body fluids as a product of protein metabolism.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.